Efficacy and safety of Qi-Wei-Qing-Yan aerosol in treatment of acute pharyngitis (lung-stomach excess-heat syndrome): study protocol for a randomized controlled trial
- PMID: 26896352
- PMCID: PMC4759740
- DOI: 10.1186/s13063-016-1217-4
Efficacy and safety of Qi-Wei-Qing-Yan aerosol in treatment of acute pharyngitis (lung-stomach excess-heat syndrome): study protocol for a randomized controlled trial
Abstract
Background: Acute pharyngitis accounts for an estimated 15 million patient visits in the United States. However, there is no proven effective and safe treatment. Although Chinese herbal medicine is widely used in the treatment of acute pharyngitis, there is a lack of evidence-based data. Despite several clinical trials conducted in this setting, no randomized placebo-controlled trial has been performed to date. This trial aims to investigate the efficacy and safety of Qi-Wei-Qing-Yan aerosol (QWQYA), a Chinese herbal prescription, compared with a placebo aerosol in the treatment of acute pharyngitis with lung-stomach excess-heat syndrome.
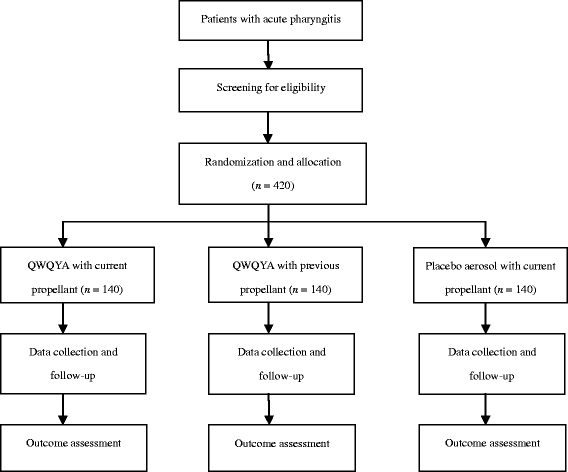
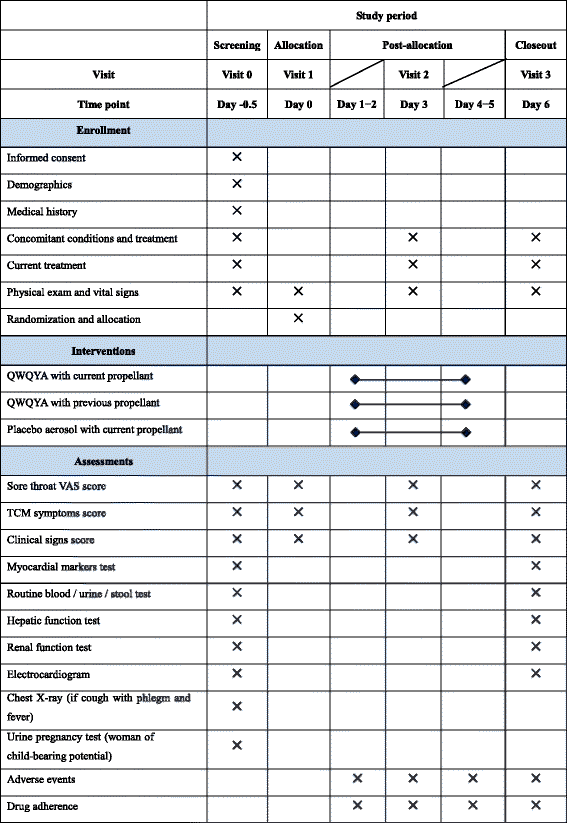
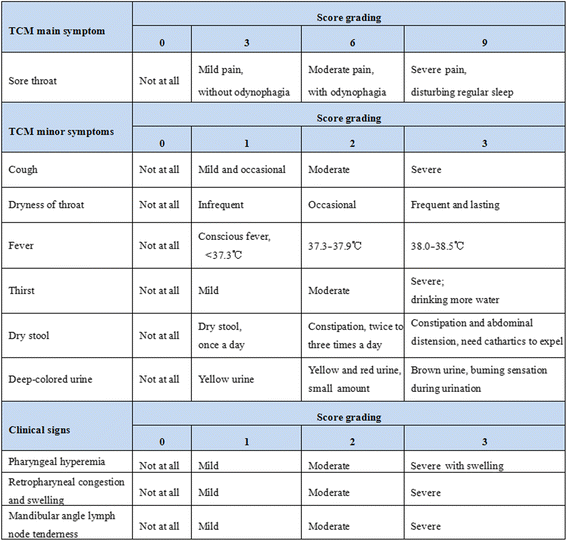
Methods/design: This is a prospective, multicenter, randomized, double-blinded, parallel-group, placebo-controlled trial. A total of 420 adult patients, of either sex, with acute pharyngitis will be enrolled from seven study sites across China. All patients will be randomly allocated to one of three parallel treatment groups: (1) QWQYA with the current propellant, (2) QWQYA with a previous propellant, and (3) the placebo aerosol with the current propellant. The study medication will be administered into the pharyngeal region in three sprays thrice daily for 5 consecutive days. The primary outcome measures are time to complete resolution of sore throat and relief rate of sore throat. Secondary outcome measures include resolution rate of sore throat, time to relief of sore throat, intensity of sore throat, and change of traditional Chinese medicine syndrome score and clinical signs score from baseline to post-treatment, as well as the occurrence of any adverse events.
Discussion: This will be the first clinical trial to investigate the efficacy and safety of QWQYA in the treatment of acute pharyngitis in an adult population in a multicenter, randomized, double-blinded, parallel-group, placebo-controlled manner. Not only might it establish the basis for the efficacy and safety of QWQYA in treating acute pharyngitis, but it might also provide evidence to support the use of Chinese herbal medicine in treating acute pharyngitis and thus support an alternative treatment option for management of acute pharyngitis.
Trial registration: Chinese Clinical Trial Registry ChiCTR-IPR-15005991.
Figures
Similar articles
-
Evaluation on immediate analgesic efficacy and safety of Kai-Hou-Jian spray (children's type) in treating sore throat caused by acute pharyngitis and tonsillitis in children: study protocol for a randomized controlled trial.Trials. 2021 Mar 18;22(1):216. doi: 10.1186/s13063-021-05148-1. Trials. 2021. PMID: 33736674 Free PMC article.
-
Safety and efficacy of a traditional herbal medicine (Throat Coat) in symptomatic temporary relief of pain in patients with acute pharyngitis: a multicenter, prospective, randomized, double-blinded, placebo-controlled study.J Altern Complement Med. 2003 Apr;9(2):285-98. doi: 10.1089/10755530360623400. J Altern Complement Med. 2003. PMID: 12804082 Clinical Trial.
-
[Study of Jinye Baidu granule in treatment of wind-warmth lung heat disease (heat in lung-wei pattern): a randomized, double-blind, parallel-controlled trial].Zhongguo Zhong Yao Za Zhi. 2017 Apr;42(8):1467-1473. doi: 10.19540/j.cnki.cjcmm.2017.0043. Zhongguo Zhong Yao Za Zhi. 2017. PMID: 29071848 Clinical Trial. Chinese.
-
Chinese medicinal herbs for sore throat.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD004877. doi: 10.1002/14651858.CD004877.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2012 Mar 14;(3):CD004877. doi: 10.1002/14651858.CD004877.pub3. PMID: 17636777 Updated. Review.
-
Chinese medicinal herbs for sore throat.Cochrane Database Syst Rev. 2012 Mar 14;2012(3):CD004877. doi: 10.1002/14651858.CD004877.pub3. Cochrane Database Syst Rev. 2012. PMID: 22419300 Free PMC article. Review.
Cited by
-
Adjunctive use of modified Yunu-Jian in the non-surgical treatment of male smokers with chronic periodontitis: a randomized double-blind, placebo-controlled clinical trial.Chin Med. 2016 Sep 20;11:40. doi: 10.1186/s13020-016-0111-z. eCollection 2016. Chin Med. 2016. PMID: 27660650 Free PMC article.
-
Yan-Hou-Qing formula attenuates ammonia-induced acute pharyngitis in rats via inhibition of NF-κB and COX-2.BMC Complement Med Ther. 2020 Sep 14;20(1):280. doi: 10.1186/s12906-020-03077-1. BMC Complement Med Ther. 2020. PMID: 32928206 Free PMC article.
-
Network Pharmacology Identifies the Mechanisms of Sang-Xing-Zhi-Ke-Fang against Pharyngitis.Evid Based Complement Alternat Med. 2020 Oct 12;2020:2421916. doi: 10.1155/2020/2421916. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33101439 Free PMC article.
References
-
- Hing E, Hall MJ, Xu J. National Hospital Ambulatory Medical Care Survey: 2006 outpatient department summary. Natl Health Stat Report. 2008; 1–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

