Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing's disease
- PMID: 26542280
- PMCID: PMC4799251
- DOI: 10.1007/s11102-015-0692-z
Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing's disease
Abstract
Purpose: In a 10-week proof-of-concept study (LINC 1), the potent oral 11β-hydroxylase inhibitor osilodrostat (LCI699) normalized urinary free cortisol (UFC) in 11/12 patients with Cushing's disease. The current 22-week study (LINC 2; NCT01331239) further evaluated osilodrostat in patients with Cushing's disease.
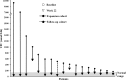
Methods: Phase II, open-label, prospective study of two patient cohorts. Follow-up cohort: 4/12 patients previously enrolled in LINC 1, offered re-enrollment if baseline mean UFC was above ULN. Expansion cohort: 15 newly enrolled patients with baseline UFC > 1.5 × ULN. In the follow-up cohort, patients initiated osilodrostat twice daily at the penultimate efficacious/tolerable dose in LINC 1; dose was adjusted as needed. In the expansion cohort, osilodrostat was initiated at 4 mg/day (10 mg/day if baseline UFC > 3 × ULN), with dose escalated every 2 weeks to 10, 20, 40, and 60 mg/day until UFC ≤ ULN. Main efficacy endpoint was the proportion of responders (UFC ≤ ULN or ≥50% decrease from baseline) at weeks 10 and 22.
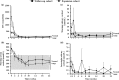
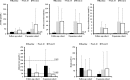
Results: Overall response rate was 89.5% (n/N = 17/19) at 10 weeks and 78.9% (n/N = 15/19) at 22 weeks; at week 22, all responding patients had UFC ≤ ULN. The most common AEs observed during osilodrostat treatment were nausea, diarrhea, asthenia, and adrenal insufficiency (n = 6 for each). New or worsening hirsutism (n = 2) and/or acne (n = 3) were reported among four female patients, all of whom had increased testosterone levels.
Conclusions: Osilodrostat treatment reduced UFC in all patients; 78.9% (n/N = 15/19) had normal UFC at week 22. Treatment with osilodrostat was generally well tolerated.
Keywords: 11β-hydroxylase; Cortisol; Cushing’s; LCI699; Osilodrostat.
Figures

Similar articles
-
Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase.Lancet Diabetes Endocrinol. 2020 Sep;8(9):748-761. doi: 10.1016/S2213-8587(20)30240-0. Epub 2020 Jul 27. Lancet Diabetes Endocrinol. 2020. PMID: 32730798 Clinical Trial.
-
LCI699, a potent 11β-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: results from a multicenter, proof-of-concept study.J Clin Endocrinol Metab. 2014 Apr;99(4):1375-83. doi: 10.1210/jc.2013-2117. Epub 2013 Dec 11. J Clin Endocrinol Metab. 2014. PMID: 24423285 Clinical Trial.
-
A multicenter, phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing's syndrome other than Cushing's disease.Endocr J. 2020 Aug 28;67(8):841-852. doi: 10.1507/endocrj.EJ19-0617. Epub 2020 May 1. Endocr J. 2020. PMID: 32378529 Clinical Trial.
-
Clinical Utility of Osilodrostat in Cushing's Disease: Review of Currently Available Literature.Drug Des Devel Ther. 2023 Apr 27;17:1303-1312. doi: 10.2147/DDDT.S315359. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37143705 Free PMC article. Review.
-
Osilodrostat for the treatment of Cushing's disease.Expert Opin Pharmacother. 2021 Jun;22(9):1099-1106. doi: 10.1080/14656566.2021.1897106. Epub 2021 Mar 11. Expert Opin Pharmacother. 2021. PMID: 33703978 Review.
Cited by
-
Fatal high-grade skull osteosarcoma 30 years following radiotherapy for Cushing's disease.Endocrinol Diabetes Metab Case Rep. 2020 Jul 5;2020:20-0062. doi: 10.1530/EDM-20-0062. Online ahead of print. Endocrinol Diabetes Metab Case Rep. 2020. PMID: 32698127 Free PMC article.
-
Cushing's disease: the burden of illness.Endocrine. 2017 Apr;56(1):10-18. doi: 10.1007/s12020-016-0984-8. Epub 2016 May 17. Endocrine. 2017. PMID: 27189147 Review.
-
Levoketoconazole in the Treatment of Patients With Cushing's Syndrome and Diabetes Mellitus: Results From the SONICS Phase 3 Study.Front Endocrinol (Lausanne). 2021 Apr 7;12:595894. doi: 10.3389/fendo.2021.595894. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33897615 Free PMC article. Clinical Trial.
-
New Insights in Cushing Disease Treatment With Focus on a Derivative of Vitamin A.Front Endocrinol (Lausanne). 2018 May 24;9:262. doi: 10.3389/fendo.2018.00262. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29881371 Free PMC article. Review.
-
Functioning Pituitary Adenomas - Current Treatment Options and Emerging Medical Therapies.Eur Endocrinol. 2019 Apr;15(1):30-40. doi: 10.17925/EE.2019.15.1.30. Epub 2019 Apr 12. Eur Endocrinol. 2019. PMID: 31244908 Free PMC article. Review.
References
-
- Biller BMK, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2008;93(7):2454–2462. doi: 10.1210/jc.2007-2734. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

