Detailed Biochemical and Bioenergetic Characterization of FBXL4-Related Encephalomyopathic Mitochondrial DNA Depletion
- PMID: 26404457
- PMCID: PMC5580732
- DOI: 10.1007/8904_2015_491
Detailed Biochemical and Bioenergetic Characterization of FBXL4-Related Encephalomyopathic Mitochondrial DNA Depletion
Abstract
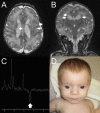
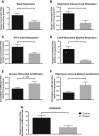
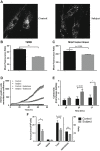
Mutations of FBXL4, which encodes an orphan mitochondrial F-box protein, are a recently identified cause of encephalomyopathic mtDNA depletion. Here, we describe the detailed clinical and biochemical phenotype of a neonate presenting with hyperlactatemia, leukoencephalopathy, arrhythmias, pulmonary hypertension, dysmorphic features, and lymphopenia. Next-generation sequencing in the proband identified a homozygous frameshift, c.1641_1642delTG, in FBXL4, with a surrounding block of SNP marker homozygosity identified by microarray. Muscle biopsy showed a paucity of mitochondria with ultrastructural abnormalities, mitochondrial DNA depletion, and profound deficiency of all respiratory chain complexes. Cell-based mitochondrial phenotyping in fibroblasts showed mitochondrial fragmentation, decreased basal and maximal respiration, absence of ATP-linked respiratory and leak capacity, impaired survival under obligate aerobic respiration, and reduced mitochondrial inner membrane potential, with relative sparing of mitochondrial mass. Cultured fibroblasts from the patient exhibited a more oxidized glutathione ratio, consistent with altered cellular redox poise. High-resolution respirometry of permeabilized muscle fibers showed marked deficiency of oxidative phosphorylation using a variety of mitochondrial energy substrates and inhibitors. This constitutes the fourth and most detailed report of FBXL4 deficiency to date. In light of our patient's clinical findings and genotype (homozygous frameshift), this phenotype likely represents the severe end of the FBXL4 clinical spectrum.
Conflict of interest statement
Ghadi Antoun, Skye McBride, Jason R. Vanstone, Turaya Naas, Jean Michaud, Stephanie Redpath, Hugh J. McMillan, Jason Brophy, Hussein Daoud, Pranesh Chakraborty, David Dyment, Martin Holcik, Mary-Ellen Harper, and Matthew A. Lines declare that they have no conflict of interest.
Figures
Similar articles
-
Molecular Characterization of New FBXL4 Mutations in Patients With mtDNA Depletion Syndrome.Front Genet. 2020 Jan 8;10:1300. doi: 10.3389/fgene.2019.01300. eCollection 2019. Front Genet. 2020. PMID: 31969900 Free PMC article.
-
A novel mutation in FBXL4 in a Norwegian child with encephalomyopathic mitochondrial DNA depletion syndrome 13.Eur J Med Genet. 2016 Jun;59(6-7):342-6. doi: 10.1016/j.ejmg.2016.05.005. Epub 2016 May 13. Eur J Med Genet. 2016. PMID: 27182039
-
Characterization of the C584R variant in the mtDNA depletion syndrome gene FBXL4, reveals a novel role for FBXL4 as a regulator of mitochondrial fusion.Biochim Biophys Acta Mol Basis Dis. 2019 Nov 1;1865(11):165536. doi: 10.1016/j.bbadis.2019.165536. Epub 2019 Aug 20. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31442532
-
Molecular and clinical spectra of FBXL4 deficiency.Hum Mutat. 2017 Dec;38(12):1649-1659. doi: 10.1002/humu.23341. Epub 2017 Oct 6. Hum Mutat. 2017. PMID: 28940506 Review.
-
Mitochondrial DNA Depletion Syndrome and Its Associated Cardiac Disease.Front Cardiovasc Med. 2022 Feb 14;8:808115. doi: 10.3389/fcvm.2021.808115. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35237671 Free PMC article. Review.
Cited by
-
FBXL4-Related Mitochondrial DNA Depletion Syndrome 13 (MTDPS13): A Case Report With a Comprehensive Mutation Review.Front Genet. 2019 Feb 5;10:39. doi: 10.3389/fgene.2019.00039. eCollection 2019. Front Genet. 2019. PMID: 30804983 Free PMC article.
-
Thirty years of translational research in Mobility Medicine: Collection of abstracts of the 2020 Padua Muscle Days.Eur J Transl Myol. 2020 Apr 1;30(1):8826. doi: 10.4081/ejtm.2019.8826. eCollection 2020 Apr 7. Eur J Transl Myol. 2020. PMID: 32499887 Free PMC article.
-
FBXL4 deficiency increases mitochondrial removal by autophagy.EMBO Mol Med. 2020 Jul 7;12(7):e11659. doi: 10.15252/emmm.201911659. Epub 2020 Jun 11. EMBO Mol Med. 2020. PMID: 32525278 Free PMC article.
-
Mitochondrial Dynamics: Molecular Mechanisms, Related Primary Mitochondrial Disorders and Therapeutic Approaches.Genes (Basel). 2021 Feb 10;12(2):247. doi: 10.3390/genes12020247. Genes (Basel). 2021. PMID: 33578638 Free PMC article. Review.
-
MPV17 Mutations Are Associated With a Quiescent Energetic Metabolic Profile.Front Cell Neurosci. 2021 Mar 17;15:641264. doi: 10.3389/fncel.2021.641264. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33815063 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

