Intravenous Artesunate for the Treatment of Severe and Complicated Malaria in the United States: Clinical Use Under an Investigational New Drug Protocol
- PMID: 26301474
- PMCID: PMC4627466
- DOI: 10.7326/M15-0910
Intravenous Artesunate for the Treatment of Severe and Complicated Malaria in the United States: Clinical Use Under an Investigational New Drug Protocol
Abstract
Background: Quinidine gluconate, the only U.S. Food and Drug Administration-approved treatment for life-threatening malaria in the United States, has a problematic safety profile and is often unavailable in hospitals.
Objective: To assess the safety and clinical benefit of intravenous artesunate as an alternative to quinidine.
Design: Retrospective case series.
Setting: U.S. hospitals.
Patients: 102 patients aged 1 to 72 years (90% adults; 61% men) with severe and complicated malaria. Patients received 4 weight-based doses of intravenous artesunate (2.4 mg/kg) under a treatment protocol implemented by the Centers for Disease Control and Prevention between January 2007 and December 2010. At baseline, 35% had evidence of cerebral malaria, and 17% had severe hepatic impairment. Eligibility required the presence of microscopically confirmed malaria, need for intravenous treatment, and an impediment to quinidine.
Measurements: Clinical and laboratory data from each patient's hospital records were abstracted retrospectively, including information from baseline through a maximum 7-day follow-up, and presented before a physician committee to evaluate safety and clinical benefit outcomes.
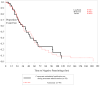
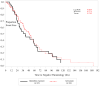
Results: 7 patients died (mortality rate, 6.9%). The most frequent adverse events were anemia (65%) and elevated hepatic enzyme levels (49%). All deaths and most adverse events were attributed to the severity of malaria. Patients' symptoms generally improved or resolved within 3 days, and the median time to discharge from the intensive care unit was 4 days, even for patients with severe liver disease or cerebral malaria. More than 100 concomitant medications were used, with no documented drug-drug interactions.
Limitation: Potential late-presenting safety issues might occur outside the 7-day follow-up.
Conclusion: Artesunate was a safe and clinically beneficial alternative to quinidine.
Figures
Similar articles
-
Artesunate: investigational drug for the treatment of severe falciparum malaria in Hawai'i.Hawaii Med J. 2011 Apr;70(4):77-9. Hawaii Med J. 2011. PMID: 21785506 Free PMC article. Review.
-
Intravenous artesunate for the treatment of severe malaria.Ann Pharmacother. 2010 Jul-Aug;44(7-8):1250-8. doi: 10.1345/aph.1M732. Epub 2010 Jun 15. Ann Pharmacother. 2010. PMID: 20551300 Review.
-
Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Colombia.Malar J. 2007 Feb 28;6:25. doi: 10.1186/1475-2875-6-25. Malar J. 2007. PMID: 17328806 Free PMC article. Clinical Trial.
-
Comparison of artesunate and quinine in the treatment of severe Plasmodium falciparum malaria at Kassala hospital, Sudan.J Infect Dev Ctries. 2014 May 14;8(5):611-5. doi: 10.3855/jidc.3813. J Infect Dev Ctries. 2014. PMID: 24820465 Clinical Trial.
-
Safety and Effectiveness of Intravenous Artesunate for Treatment of Severe Malaria in the United States-April 2019 Through December 2020.Clin Infect Dis. 2021 Dec 6;73(11):1965-1972. doi: 10.1093/cid/ciab570. Clin Infect Dis. 2021. PMID: 34314501 Free PMC article.
Cited by
-
Response to Anastasio et al. - Severe imported falciparum malaria - Clinical and drug supply challenges.Travel Med Infect Dis. 2019 Jan-Feb;27:116. doi: 10.1016/j.tmaid.2018.11.003. Epub 2018 Nov 8. Travel Med Infect Dis. 2019. PMID: 30415080 Free PMC article. No abstract available.
-
Expanded Availability of Intravenous Artesunate for the Treatment of Severe Malaria in the United States.Am J Trop Med Hyg. 2019 Jun;100(6):1295-1296. doi: 10.4269/ajtmh.19-0230. Am J Trop Med Hyg. 2019. PMID: 30927931 Free PMC article. No abstract available.
-
Malaria in Children.Infect Dis Clin North Am. 2018 Mar;32(1):189-200. doi: 10.1016/j.idc.2017.10.008. Epub 2017 Dec 18. Infect Dis Clin North Am. 2018. PMID: 29269188 Free PMC article. Review.
-
A Case of Plasmodium falciparum Malaria Treated with Artesunate in a 55-Year-Old Woman on Return to Florida from a Visit to Ghana.Am J Case Rep. 2020 Dec 20;21:e926097. doi: 10.12659/AJCR.926097. Am J Case Rep. 2020. PMID: 33341821 Free PMC article.
-
Delayed autoimmune haemolytic anaemia after artesunate therapy for severe malaria.BMJ Case Rep. 2022 Jan 17;15(1):e245845. doi: 10.1136/bcr-2021-245845. BMJ Case Rep. 2022. PMID: 35039352 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) Malaria Facts. Updated: March 26, 2014. Available at : http://www.cdc.gov/malaria/about/facts.html. Accessed: October 14, 2014.
-
- World Health Organization (WHO) Guidelines for the Treatment of Malaria. 2nd World Health Organization; Geneva: 2010. pp. 35–37.
-
- WHO Severe Falciparum malaria. Transactions of the Royal Society of Tropical Medicinne and Hygiene. 2000;94(Suppl 1):1–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
