A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia
- PMID: 25852082
- PMCID: PMC4490203
- DOI: 10.1093/cvr/cvv127
A novel human R25C-phospholamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia
Abstract
Aims: Depressed sarcoplasmic reticulum (SR) Ca(2+) cycling, a universal characteristic of human and experimental heart failure, may be associated with genetic alterations in key Ca(2+)-handling proteins. In this study, we identified a novel PLN mutation (R25C) in dilated cardiomyopathy (DCM) and investigated its functional significance in cardiomyocyte Ca(2+)-handling and contractility.
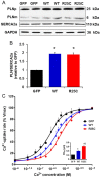
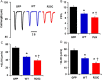
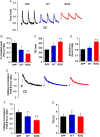
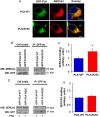
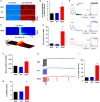
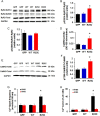
Methods and results: Exome sequencing identified a C73T substitution in the coding region of PLN in a family with DCM. The four heterozygous family members had implantable cardiac defibrillators, and three developed prominent ventricular arrhythmias. Overexpression of R25C-PLN in adult rat cardiomyocytes significantly suppressed the Ca(2+) affinity of SR Ca(2+)-ATPase (SERCA2a), resulting in decreased SR Ca(2+) content, Ca(2+) transients, and impaired contractile function, compared with WT-PLN. These inhibitory effects were associated with enhanced interaction of R25C-PLN with SERCA2, which was prevented by PKA phosphorylation. Accordingly, isoproterenol stimulation relieved the depressive effects of R25C-PLN in cardiomyocytes. However, R25C-PLN also elicited increases in the frequency of Ca(2+) sparks and waves as well as stress-induced aftercontractions. This was accompanied by increased Ca(2+)/calmodulin-dependent protein kinase II activity and hyper-phosphorylation of RyR2 at serine 2814.
Conclusion: The findings demonstrate that human R25C-PLN is associated with super-inhibition of SERCA2a and Ca(2+) transport as well as increased SR Ca(2+) leak, promoting arrhythmogenesis under stress conditions. This is the first mechanistic evidence that increased PLN inhibition may impact both SR Ca(2+) uptake and Ca(2+) release activities and suggests that the human R25C-PLN may be a prognostic factor for increased ventricular arrhythmia risk in DCM carriers.
Keywords: Calcium cycling; Dilated cardiomyopathy; Mutation.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author 2015. For permissions please email: journals.permissions@oup.com.
Figures
Comment in
-
Linking superinhibitory PLN mutations to CaMKII activation: a new arrhythmogenic mechanism in genetic DCM?Cardiovasc Res. 2015 Jul 1;107(1):5-6. doi: 10.1093/cvr/cvv163. Epub 2015 May 26. Cardiovasc Res. 2015. PMID: 26014576 No abstract available.
Similar articles
-
Phospholamban ablation rescues the enhanced propensity to arrhythmias of mice with CaMKII-constitutive phosphorylation of RyR2 at site S2814.J Physiol. 2016 Jun 1;594(11):3005-30. doi: 10.1113/JP271622. Epub 2016 Feb 2. J Physiol. 2016. PMID: 26695843 Free PMC article.
-
Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors.Cardiovasc Res. 2019 Mar 1;115(3):556-569. doi: 10.1093/cvr/cvy213. Cardiovasc Res. 2019. PMID: 30169578 Free PMC article.
-
Phospholamban ablation rescues sarcoplasmic reticulum Ca(2+) handling but exacerbates cardiac dysfunction in CaMKIIdelta(C) transgenic mice.Circ Res. 2010 Feb 5;106(2):354-62. doi: 10.1161/CIRCRESAHA.109.207423. Epub 2009 Dec 3. Circ Res. 2010. PMID: 19959778 Free PMC article.
-
Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend.J Mol Cell Cardiol. 2014 Dec;77:160-7. doi: 10.1016/j.yjmcc.2014.10.005. Epub 2014 Oct 18. J Mol Cell Cardiol. 2014. PMID: 25451386 Free PMC article. Review.
-
Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy.Biochem Biophys Res Commun. 2004 Oct 1;322(4):1214-22. doi: 10.1016/j.bbrc.2004.07.164. Biochem Biophys Res Commun. 2004. PMID: 15336969 Review.
Cited by
-
Impairment of the ER/mitochondria compartment in human cardiomyocytes with PLN p.Arg14del mutation.EMBO Mol Med. 2021 Jun 7;13(6):e13074. doi: 10.15252/emmm.202013074. Epub 2021 May 16. EMBO Mol Med. 2021. PMID: 33998164 Free PMC article.
-
Phospholamban regulates nuclear Ca2+ stores and inositol 1,4,5-trisphosphate mediated nuclear Ca2+ cycling in cardiomyocytes.J Mol Cell Cardiol. 2018 Oct;123:185-197. doi: 10.1016/j.yjmcc.2018.09.008. Epub 2018 Sep 24. J Mol Cell Cardiol. 2018. PMID: 30261161 Free PMC article.
-
Aberrant PLN-R14del Protein Interactions Intensify SERCA2a Inhibition, Driving Impaired Ca2+ Handling and Arrhythmogenesis.Int J Mol Sci. 2022 Jun 22;23(13):6947. doi: 10.3390/ijms23136947. Int J Mol Sci. 2022. PMID: 35805951 Free PMC article.
-
Phospholamban p.Arg14del Cardiomyopathy: A Japanese Case Series.Intern Med. 2022 Jul 1;61(13):1987-1993. doi: 10.2169/internalmedicine.8594-21. Epub 2021 Dec 18. Intern Med. 2022. PMID: 34924461 Free PMC article.
-
Phosphorylation of cardiac myosin-binding protein-C contributes to calcium homeostasis.J Biol Chem. 2020 Aug 7;295(32):11275-11291. doi: 10.1074/jbc.RA120.013296. Epub 2020 Jun 18. J Biol Chem. 2020. PMID: 32554466 Free PMC article.
References
-
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol 2013;10:531–547. - PubMed
-
- Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 1992;85:1046–1055. - PubMed
-
- Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 1987;61:70–76. - PubMed
-
- Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res 1998;37:279–289. - PubMed
-
- Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 1995;92:778–784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

