Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects
- PMID: 25789548
- PMCID: PMC5306451
- DOI: 10.1165/rcmb.2014-0483OC
Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects
Abstract
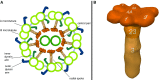
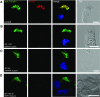
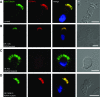
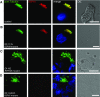
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous recessive disorder caused by several distinct defects in genes responsible for ciliary beating, leading to defective mucociliary clearance often associated with randomization of left/right body asymmetry. Individuals with PCD caused by defective radial spoke (RS) heads are difficult to diagnose owing to lack of gross ultrastructural defects and absence of situs inversus. Thus far, most mutations identified in human radial spoke genes (RSPH) are loss-of-function mutations, and missense variants have been rarely described. We studied the consequences of different RSPH9, RSPH4A, and RSPH1 mutations on the assembly of the RS complex to improve diagnostics in PCD. We report 21 individuals with PCD (16 families) with biallelic mutations in RSPH9, RSPH4A, and RSPH1, including seven novel mutations comprising missense variants, and performed high-resolution immunofluorescence analysis of human respiratory cilia. Missense variants are frequent genetic defects in PCD with RS defects. Absence of RSPH4A due to mutations in RSPH4A results in deficient axonemal assembly of the RS head components RSPH1 and RSPH9. RSPH1 mutant cilia, lacking RSPH1, fail to assemble RSPH9, whereas RSPH9 mutations result in axonemal absence of RSPH9, but do not affect the assembly of the other head proteins, RSPH1 and RSPH4A. Interestingly, our results were identical in individuals carrying loss-of-function mutations, missense variants, or one amino acid deletion. Immunofluorescence analysis can improve diagnosis of PCD in patients with loss-of-function mutations as well as missense variants. RSPH4A is the core protein of the RS head.
Keywords: cilia; human radial spoke protein 1; human radial spoke protein 4A; human radial spoke protein 9; primary ciliary dyskinesia.
Figures


Similar articles
-
Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects.Hum Mol Genet. 2014 Jul 1;23(13):3362-74. doi: 10.1093/hmg/ddu046. Epub 2014 Feb 11. Hum Mol Genet. 2014. PMID: 24518672 Free PMC article.
-
Mutations in radial spoke head genes and ultrastructural cilia defects in East-European cohort of primary ciliary dyskinesia patients.PLoS One. 2012;7(3):e33667. doi: 10.1371/journal.pone.0033667. Epub 2012 Mar 20. PLoS One. 2012. PMID: 22448264 Free PMC article.
-
Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities.Am J Hum Genet. 2009 Feb;84(2):197-209. doi: 10.1016/j.ajhg.2009.01.011. Epub 2009 Feb 5. Am J Hum Genet. 2009. PMID: 19200523 Free PMC article.
-
Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure.Ultrastruct Pathol. 2017 Nov-Dec;41(6):373-385. doi: 10.1080/01913123.2017.1362088. Epub 2017 Sep 15. Ultrastruct Pathol. 2017. PMID: 28915070 Free PMC article. Review.
-
Primary Ciliary Dyskinesia.2007 Jan 24 [updated 2019 Dec 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2007 Jan 24 [updated 2019 Dec 5]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301301 Free Books & Documents. Review.
Cited by
-
Rsph4a is essential for the triplet radial spoke head assembly of the mouse motile cilia.PLoS Genet. 2020 Mar 23;16(3):e1008664. doi: 10.1371/journal.pgen.1008664. eCollection 2020 Mar. PLoS Genet. 2020. PMID: 32203505 Free PMC article.
-
Structure of the radial spoke head and insights into its role in mechanoregulation of ciliary beating.Nat Struct Mol Biol. 2021 Jan;28(1):20-28. doi: 10.1038/s41594-020-00519-9. Epub 2020 Dec 14. Nat Struct Mol Biol. 2021. PMID: 33318704 Free PMC article.
-
Mutations in C11orf70 Cause Primary Ciliary Dyskinesia with Randomization of Left/Right Body Asymmetry Due to Defects of Outer and Inner Dynein Arms.Am J Hum Genet. 2018 May 3;102(5):973-984. doi: 10.1016/j.ajhg.2018.03.025. Am J Hum Genet. 2018. PMID: 29727693 Free PMC article.
-
Proteomic Analysis of Primary Human Airway Epithelial Cells Exposed to the Respiratory Toxicant Diacetyl.J Proteome Res. 2017 Feb 3;16(2):538-549. doi: 10.1021/acs.jproteome.6b00672. Epub 2017 Jan 9. J Proteome Res. 2017. PMID: 27966365 Free PMC article.
-
Distinct architecture and composition of mouse axonemal radial spoke head revealed by cryo-EM.Proc Natl Acad Sci U S A. 2021 Jan 26;118(4):e2021180118. doi: 10.1073/pnas.2021180118. Proc Natl Acad Sci U S A. 2021. PMID: 34871179 Free PMC article.
References
-
- Ibañez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 2003;12:R27–R35. - PubMed
-
- Marthin JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med. 2010;181:1262–1268. - PubMed
-
- Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol. 2007;69:423–450. - PubMed
-
- Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–2821. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

