Ganglioside GM3 is essential for the structural integrity and function of cochlear hair cells
- PMID: 25652401
- PMCID: PMC4425844
- DOI: 10.1093/hmg/ddv041
Ganglioside GM3 is essential for the structural integrity and function of cochlear hair cells
Abstract
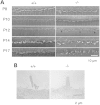
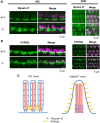
GM3 synthase (ST3GAL5) is the first biosynthetic enzyme of a- and b-series gangliosides. Patients with GM3 synthase deficiency suffer severe neurological disability and deafness. Eight children (ages 4.1 ± 2.3 years) homozygous for ST3GAL5 c.694C>T had no detectable GM3 (a-series) or GD3 (b-series) in plasma. Their auditory function was characterized by the absence of middle ear muscle reflexes, distortion product otoacoustic emissions and cochlear microphonics, as well as abnormal auditory brainstem responses and cortical auditory-evoked potentials. In St3gal5(-/-) mice, stereocilia of outer hair cells showed signs of degeneration as early as postnatal Day 3 (P3); thereafter, blebs devoid of actin or tubulin appeared at the region of vestigial kinocilia, suggesting impaired vesicular trafficking. Stereocilia of St3gal5(-/-) inner hair cells were fused by P17, and protein tyrosine phosphatase receptor Q, normally linked to myosin VI at the tapered base of stereocilia, was maldistributed along the cell membrane. B4galnt1(-/-) (GM2 synthase-deficient) mice expressing only GM3 and GD3 gangliosides had normal auditory structure and function. Thus, GM3-dependent membrane microdomains might be essential for the proper organization and maintenance of stereocilia in auditory hair cells.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
Similar articles
-
Gangliosides and hearing.Biochim Biophys Acta Gen Subj. 2017 Oct;1861(10):2485-2493. doi: 10.1016/j.bbagen.2017.05.025. Epub 2017 May 30. Biochim Biophys Acta Gen Subj. 2017. PMID: 28571946
-
Early growth and development impairments in patients with ganglioside GM3 synthase deficiency.Clin Genet. 2016 May;89(5):625-9. doi: 10.1111/cge.12703. Epub 2016 Jan 20. Clin Genet. 2016. PMID: 26649472
-
Enhanced Susceptibility to Chemoconvulsant-Induced Seizures in Ganglioside GM3 Synthase Knockout Mice.ASN Neuro. 2020 Jan-Dec;12:1759091420938175. doi: 10.1177/1759091420938175. ASN Neuro. 2020. PMID: 32664815 Free PMC article.
-
Physiopathological function of hematoside (GM3 ganglioside).Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(4):179-98. doi: 10.2183/pjab.87.179. Proc Jpn Acad Ser B Phys Biol Sci. 2011. PMID: 21558756 Free PMC article. Review.
-
Diseases of ganglioside biosynthesis: An expanding group of congenital disorders of glycosylation.Mol Genet Metab. 2018 Aug;124(4):230-237. doi: 10.1016/j.ymgme.2018.06.014. Epub 2018 Jun 28. Mol Genet Metab. 2018. PMID: 29983310 Review.
Cited by
-
Gangliosides in Neurodegenerative Diseases.Adv Neurobiol. 2023;29:391-418. doi: 10.1007/978-3-031-12390-0_13. Adv Neurobiol. 2023. PMID: 36255682
-
Gangliosides in the Brain: Physiology, Pathophysiology and Therapeutic Applications.Front Neurosci. 2020 Oct 6;14:572965. doi: 10.3389/fnins.2020.572965. eCollection 2020. Front Neurosci. 2020. PMID: 33117120 Free PMC article. Review.
-
Ganglioside GM3 Synthase Deficiency in Mouse Models and Human Patients.Int J Mol Sci. 2022 May 11;23(10):5368. doi: 10.3390/ijms23105368. Int J Mol Sci. 2022. PMID: 35628171 Free PMC article. Review.
-
Neural-specific alterations in glycosphingolipid biosynthesis and cell signaling associated with two human ganglioside GM3 synthase deficiency variants.Hum Mol Genet. 2023 Dec 1;32(24):3323-3341. doi: 10.1093/hmg/ddad146. Hum Mol Genet. 2023. PMID: 37676252 Free PMC article.
-
Roles of Gangliosides in Hypothalamic Control of Energy Balance: New Insights.Int J Mol Sci. 2020 Jul 28;21(15):5349. doi: 10.3390/ijms21155349. Int J Mol Sci. 2020. PMID: 32731387 Free PMC article. Review.
References
-
- Roberts W.M., Howard J., Hudspeth A.J. (1988) Hair cells: transduction, tuning, and transmission in the inner ear. Annu. Rev. Cell Biol., 4, 63–92. - PubMed
-
- Hudspeth A.J. (1997) How hearing happens. Neuron, 19, 947–950. - PubMed
-
- Hakomori S. (1981) Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu. Rev. Biochem., 50, 733–764. - PubMed
-
- Schengrund C.L. (1990) The role(s) of gangliosides in neural differentiation and repair: a perspective. Brain. Res. Bull., 24, 131–141. - PubMed
-
- Kawai H., Allende M.L., Wada R., Kono M., Sango K., Deng C., Miyakawa T., Crawley J.N., Werth N., Bierfreund U., Sandhoff K., Proia R.L. (2001) Mice expressing only monosialoganglioside GM3 exhibit lethal audiogenic seizures. J. Biol. Chem., 276, 6885–6888. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

