Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients
- PMID: 25568981
- PMCID: PMC6517008
- DOI: 10.1002/14651858.CD010225.pub2
Single induction dose of etomidate versus other induction agents for endotracheal intubation in critically ill patients
Abstract
Background: The use of etomidate for emergency airway interventions in critically ill patients is very common. In one large registry trial, etomidate was the most commonly used agent for this indication. Etomidate is known to suppress adrenal gland function, but it remains unclear whether or not this adrenal gland dysfunction affects mortality.
Objectives: The primary objective was to assess, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects mortality.The secondary objectives were to address, in populations of critically ill patients, whether a single induction dose of etomidate for emergency airway intervention affects adrenal gland function, organ dysfunction, or health services utilization (as measured by intensive care unit (ICU) length of stay (LOS), duration of mechanical ventilation, or vasopressor requirements).We repeated analyses within subgroups defined by the aetiologies of critical illness, timing of adrenal gland function measurement, and the type of comparator drug used.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; CINAHL; EMBASE; LILACS; International Pharmaceutical Abstracts; Web of Science; the Database of Abstracts of Reviews of Effects (DARE); and ISI BIOSIS Citation index(SM) on 8 February 2013. We reran the searches in August 2014. We will deal with any studies of interest when we update the review.We also searched the Scopus database of dissertations and conference proceedings and the US Food and Drug Administration Database. We handsearched major emergency medicine, critical care, and anaesthesiology journals.We handsearched the conference proceedings of major emergency medicine, anaesthesia, and critical care conferences from 1990 to current, and performed a grey literature search of the following: Current Controlled Trials; National Health Service - The National Research Register; ClinicalTrials.gov; NEAR website.
Selection criteria: We included randomized controlled trials in patients undergoing emergency endotracheal intubation for critical illness, including but not limited to trauma, stroke, myocardial infarction, arrhythmia, septic shock, hypovolaemic or haemorrhagic shock, and undifferentiated shock states. We included single (bolus) dose etomidate for emergency airway intervention compared to any other rapid-acting intravenous bolus single-dose induction agent.
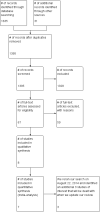
Data collection and analysis: Refinement of our initial search results by title review, and then by abstract review was carried out by three review authors. Full-text review of potential studies was based on their adherence to our inclusion and exclusion criteria. This was decided by three independent review authors. We reported the decisions regarding inclusion and exclusion in accordance with the PRISMA statement.Electronic database searching yielded 1635 potential titles, and our grey literature search yielded an additional 31 potential titles. Duplicate titles were filtered leaving 1395 titles which underwent review of their titles and abstracts by three review authors. Sixty seven titles were judged to be relevant to our review, however only eight met our inclusion criteria and seven were included in our analysis.
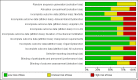
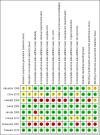
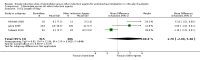
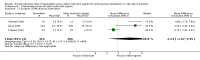
Main results: We included eight studies in the review and seven in the meta-analysis. Of those seven studies, only two were judged to be at low risk of bias. Overall, no strong evidence exists that etomidate increases mortality in critically ill patients when compared to other bolus dose induction agents (odds ratio (OR) 1.17; 95% confidence interval (CI) 0.86 to 1.60, 6 studies, 772 participants, moderate quality evidence). Due to a large number of participants lost to follow-up, we performed a post hoc sensitivity analysis. This gave a similar result (OR 1.15; 95% CI 0.86 to 1.53). There was evidence that the use of etomidate in critically ill patients was associated with a positive adrenocorticotropic hormone (ACTH) stimulation test, and this difference was more pronounced at between 4 to 6 hours (OR 19.98; 95% CI 3.95 to 101.11) than after 12 hours (OR 2.37; 95% CI 1.61 to 3.47) post-dosing. Etomidate's use in critically ill patients was associated with a small increase in SOFA score, indicating a higher risk of multisystem organ failure (mean difference (MD) 0.70; 95% CI 0.01 to 1.39, 2 studies, 591 participants, high quality evidence), but this difference was not clinically meaningful. Etomidate use did not have an effect on ICU LOS (MD 1.70 days; 95% CI -2.00 to 5.40, 4 studies, 621 participants, moderate quality evidence), hospital LOS (MD 2.41 days; 95% CI -7.08 to 11.91, 3 studies, 152 participants, moderate quality evidence), duration of mechanical ventilation (MD 2.14 days; 95% CI -1.67 to 5.95, 3 studies, 621 participants, moderate quality evidence), or duration of vasopressor use (MD 1.00 day; 95% CI -0.53 to 2.53, 1 study, 469 participants).
Authors' conclusions: Although we have not found conclusive evidence that etomidate increases mortality or healthcare resource utilization in critically ill patients, it does seem to increase the risk of adrenal gland dysfunction and multi-organ system dysfunction by a small amount. The clinical significance of this finding is unknown. This evidence is judged to be of moderate quality, owing mainly to significant attrition bias in some of the smaller studies, and new research may influence the outcomes of our review. The applicability of these data may be limited by the fact that 42% of the patients in our review were intubated for "being comatose", a population less likely to benefit from the haemodynamic stability inherent in etomidate use, and less at risk from its potential negative downstream effects of adrenal suppression.
Conflict of interest statement
Eric Bruder: none known.
Ian Ball: none known.
Stacy Ridi: none known.
William Pickett: none known.
Corinne Hohl: Dr Hohl is a full‐time faculty member with the Department of Emergency Medicine at the University of British Columbia. She has received reimbursement for travel expenses by the Canadian Association of Emergency Physicians to present on this topic in Montreal, 2010.
Figures










Update of
- doi: 10.1002/14651858.CD010225
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation.Cochrane Database Syst Rev. 2014 Jul 9;2014(7):CD009176. doi: 10.1002/14651858.CD009176.pub2. Cochrane Database Syst Rev. 2014. PMID: 25005604 Free PMC article. Review.
-
Protocol-directed sedation versus non-protocol-directed sedation to reduce duration of mechanical ventilation in mechanically ventilated intensive care patients.Cochrane Database Syst Rev. 2015 Jan 7;1:CD009771. doi: 10.1002/14651858.CD009771.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Nov 12;11:CD009771. doi: 10.1002/14651858.CD009771.pub3. PMID: 25562750 Updated. Review.
-
Protocol-directed sedation versus non-protocol-directed sedation in mechanically ventilated intensive care adults and children.Cochrane Database Syst Rev. 2018 Nov 12;11(11):CD009771. doi: 10.1002/14651858.CD009771.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480753 Free PMC article.
-
Laryngeal mask airway versus endotracheal tube for percutaneous dilatational tracheostomy in critically ill adult patients.Cochrane Database Syst Rev. 2014 Jun 30;2014(6):CD009901. doi: 10.1002/14651858.CD009901.pub2. Cochrane Database Syst Rev. 2014. PMID: 24979320 Free PMC article. Review.
Cited by
-
Comparison of induction agents for rapid sequence intubation in refractory status epilepticus: A single-center retrospective analysis.Epilepsy Behav Rep. 2024 Jan 8;25:100645. doi: 10.1016/j.ebr.2024.100645. eCollection 2024. Epilepsy Behav Rep. 2024. PMID: 38299124 Free PMC article.
-
Tertiary peritonitis: considerations for complex team-based care.Eur J Trauma Emerg Surg. 2022 Apr;48(2):811-825. doi: 10.1007/s00068-021-01750-9. Epub 2021 Jul 24. Eur J Trauma Emerg Surg. 2022. PMID: 34302503 Free PMC article. Review.
-
How Drug Shortages Affect Clinical Care: The Case of the Surgical Anesthetic Propofol.Hosp Pharm. 2015 Oct;50(9):798-805. doi: 10.1310/hpj5009-798. Epub 2015 Oct 14. Hosp Pharm. 2015. PMID: 26912921 Free PMC article.
-
Ketamine is not associated with more post-intubation hypotension than etomidate in patients undergoing endotracheal intubation.Am J Emerg Med. 2022 Nov;61:131-136. doi: 10.1016/j.ajem.2022.08.054. Epub 2022 Sep 5. Am J Emerg Med. 2022. PMID: 36096015 Free PMC article.
-
A Nomogram Model for Post-Intubation Hypotension in Patients with Severe Pneumonia in the Emergency Department.J Inflamm Res. 2023 Nov 13;16:5221-5233. doi: 10.2147/JIR.S430488. eCollection 2023. J Inflamm Res. 2023. PMID: 38026236 Free PMC article.
References
References to studies included in this review
Absalom 1999 {published data only}
-
- Absalom A, Pledger D, Kong A. Adrenocortical function in critically ill patients 24 h after a single dose of etomidate. Anaesthesia 1999;54(9):861‐7. [PUBMED: 10460557] - PubMed
-
- Absalom AR, Pledger DR, Kong A. Effects of a single dose of etomidate on adreno‐cortical function in the critically ill. British Journal of Anaesthesia 1997;79 (5):679P. - PubMed
Cinar 2010 {published data only}
-
- Cinar O, Pirat A, Zeyneloglu P, Bayraktar N, Arslan G. Hemodynamic and metabolic responses to ketamine and etomidate sedations during endotracheal intubation in critically ill patients. Critical Care Medicine. Conference: 40th Critical Care Congress of the Society of Critical Care Medicine San Diego, CA United States. San Diego, CA United States, 2010; Vol. 38 (12 Suppl).
Hildreth 2008 {published data only}
-
- Hildreth AN, Mejia VA, Maxwell RA, Smith PW, Dart BW, Barker DE. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. Journal of Trauma Injury, Infection, and Critical Care 2008;65(3):573‐8. [PUBMED: 18784570] - PubMed
Jabre 2009 {published data only}
-
- Jabre P, Combes X, Lapostolle F, Dhaouadi M, Ricard‐Hibon A, Vivien B, et al. Etomidate versus ketamine for rapid sequence intubation in acutely ill patients: a multicentre randomised controlled trial. Lancet 2009;374(9686):293‐300. [PUBMED: 19573904] - PubMed
Jacoby 2006 {published data only}
-
- Jacoby J, Heller M, Nicholas J, Patel N, Cesta M, Smith G, et al. Etomidate Versus Midazolam for Out‐of‐Hospital Intubation: A Prospective, Randomized Trial. Annals of Emergency Medicine 2006;47(6):525‐30. [PUBMED: 16713778] - PubMed
-
- Jacoby JL, Cesta M, McGee J, Heller MB, Reed J. Etomidate versus midazolam for out‐of hospital Intubation: A prospective randomized trial ‐ Abstract 175 ACEP Research Forum 2003. Annals Of Emergency Medicine 2003;42(4):S48. - PubMed
Koksal 2013 {unpublished data only}
-
- Koksal G. The effect of single dose of etomidate used during emergency intubation on hemodynamics and adrenal cortex. Unpublished manuscript N/A. - PubMed
Schenarts 2001 {published data only}
-
- Schenarts CL, Burton JH, Riker RR. Adrenocortical dysfunction following etomidate induction in emergency department patients. Academic Emergency Medicine 2001;8(1):1‐7. [PUBMED: 11136139] - PubMed
-
- Schenarts CL, Riker RR. 1999 SAEM Annual Meeting abstracts. Abstract 449: Adrenocortical dysfunction following etomidate induction in emergency department. Academic Emergency Medicine 1999;6(5):520. - PubMed
Tekwani 2010 {published data only}
-
- Tekwani K, Watts H, Sweis R, Rzechula K, Kulstad E. The effect of etomidate on hospital length of stay of patients with sepsis: A prospective, randomized study: Conference: American College of Emergency Physicians, ACEP 2009 Research Forum. Annals of Emergency Medicine 2009;54(3):S4. - PubMed
-
- Tekwani KL, Watts HF, Sweis RT, Rzechula KH, Kulstad EB. A comparison of the effects of etomidate and midazolam on hospital length of stay in patients with suspected sepsis: a prospective, randomized study. Annals of Emergency Medicine 2010;56(5):481‐9. [PUBMED: 20828877] - PubMed
References to studies excluded from this review
Asehnoune 2012 {published data only}
-
- Asehnoune K, Mahe PJ, Seguin P, Jaber S, Jung B, Guitton C, et al. Etomidate increases susceptibility to pneumonia in trauma patients. Intensive Care Medicine 2012;38(10):1673‐82. [PUBMED: 22777514] - PubMed
Baird 2009 {published data only}
-
- Baird CR, Hay AW, McKeown DW, Ray DC. Rapid sequence induction in the emergency department: induction drug and outcome of patients admitted to the intensive care unit. Emergency Medicine Journal 2009;26(8):576‐9. [PUBMED: 19625554] - PubMed
Borner 1985 {published data only}
-
- Borner U, Gips H, Boldt J, Hoge R, Bormann B, Hempelmann G. [Effect of an introductory dose of etomidate, methohexital and midazolam on adrenal cortex function before and after ACTH‐stimulation]. Deutsche Medizinische Wochenschrift 1985;10(19):750‐2. - PubMed
Bramwell 2002 {published data only}
-
- Bramwell KJ, Haizlip J, Pribble C, VanDerHeyden TC, Witte M. The effect of etomidate on intracranial pressure, mean arterial pressure, and cerebral perfusion pressure in pediatric patients with severe traumatic brain injury. Annals of Emergency Medicine 2002;40(4):S22. - PubMed
Cherfan 2011 {published data only}
Cotton 2008 {published data only}
-
- Cotton BA, Guillamondegui OD, Fleming SB, Carpenter RO, Patel SH, Morris JA Jr, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients. Archives of Surgery 2008;143(1):62‐7. [PUBMED: 18209154] - PubMed
Cuthbertson 2009 {published data only}
-
- Cuthbertson BH, Sprung CL, Annane D, Chevret S, Garfield M, Goodman S, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Medicine 2009;35(11):1868‐76. [PUBMED: 19652948] - PubMed
den Brinker 2008 {published data only}
Dmello 2010 {published data only}
-
- Dmello D, Taylor S, O'Brien J, Matuschak GM. Outcomes of etomidate in severe sepsis and septic shock. Chest 2010;138(6):1327‐32. [PUBMED: 20651024] - PubMed
Ehrman 2010 {published data only}
-
- Ehrman R, Wira C, Hayward A, Lomax A, Mullen M. Etomidate use In sepsis does not increase mortality. Annals of Emergency Medicine 2010;56(3):S117.
McPhee 2013 {published data only}
-
- McPhee LC, Badawi O, Fraser GL, Lerwick PA, Riker RR, Zuckerman IH, et al. Single‐dose etomidate is not associated with increased mortality in ICU patients with sepsis: analysis of a large electronic ICU database. Critical Care Medicine 2013;41(3):774‐83. [PUBMED: 23318491] - PubMed
Mohammad 2006 {published data only}
Morel 2011 {published data only}
-
- Morel J, Salard M, Castelain C, Bayon MC, Lambert P, Vola M, et al. Haemodynamic consequences of etomidate administration in elective cardiac surgery: a randomized double‐blinded study. British Journal of Anaesthesia 2011;107(4):503‐9. [PUBMED: 21685487 ] - PubMed
Ray 2007 {published data only}
Vinclair 2008 {published data only}
-
- Vinclair M, Broux C, Faure P, Brun J, Genty C, Jacquot C, et al. Duration of adrenal inhibition following a single dose of etomidate in critically ill patients. Intensive Care Medicine 2008;34(4):714‐9. [PUBMED: 18092151] - PubMed
Warner Kier 2009 {published data only}
-
- Warner KJ, Cuschieri J, Jurkovich GJ, Bulger EM. Single‐dose etomidate for rapid sequence intubation may impact outcome after severe injury. Journal of Trauma Injury, Infection, and Critical Care 2009;67(1):45‐50. [PUBMED: 19590307] - PubMed
Zed 2006 {published data only}
-
- Zed PJ, Abu‐Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Academic Emergency Medicine 2006;13(4):378‐83. [PUBMED: 16531603] - PubMed
References to studies awaiting assessment
Driver 2014 {published data only}
-
- Driver B, Moore J, Reardon R, Steinberg L, Antolick A, Usher S, Miner J. Ketamine versus etomidate for ED rapid sequence intubation. Academic Emergency Medicine 2014;21: 5 Suppl 1:S117.
Freund 2014 {published data only}
-
- Freund Y, Jabre P, Mourad J, Lapostolle F, Reuter PG, Woimant M, et al. Relative adrenal insufficiency in critically ill patient after rapid sequence intubation: KETASED ancillary study. Journal of Critical Care 2014;29(3):386‐9. - PubMed
Punt 2014 {published data only}
-
- Punt CD, Dormans TPJ, Oosterhuis WP, Boer W, Depoorter B, Linden CJ, et al. Etomidate and S‐ketamine for the intubation of patients on the intensive care unit: A prospective, open‐label study. Netherlands Journal of Critical Care 2014;18(2):4‐7.
Additional references
Albert 2011
-
- Albert SG, Ariyan S, Rather A. The effect of etomidate on adrenal function in critical illness: a systematic review. Intensive Care Medicine 2011;37(6):901‐10. [PUBMED: 21373823] - PubMed
Annane 2002
-
- Annane D, Sébille V, Charpentier C, Bollaert PE, François B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA 2002;288:862‐71. [MEDLINE: ] - PubMed
Annane 2004
Chan 2012
-
- Chan CM, Mitchell AL, Shorr AF. Etomidate is associated with mortality and adrenal insufficiency in sepsis: a meta‐analysis. Critical Care Medicine 2012;40(11):2945‐53. [PUBMED: 22971586] - PubMed
Cronin 1995
-
- Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, et al. Corticosteroid treatment for sepsis: a critical appraisal and meta‐analysis of the literature. Critical Care Medicine 1995;23:1430‐9. [MEDLINE: ] - PubMed
de Jong 1984
-
- Jong FH, Mallios C, Jansen C, Scheck PA, Lamberts SW. Etomidate suppresses adrenocortical function by inhibition of 11 beta‐hydroxylation. Journal of Clinical Endocrinology and Metabolism 2084;59:1143‐7. [MEDLINE: ] - PubMed
Egger 1997
Guyatt 2008
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hildreth 2013 [pers comm]
-
- Hildreth A. Re: stim test results [personal communication]. E‐mail to: E Bruder 13 November, 2013.
Hohl 2010
-
- Hohl CM, Kelly‐Smith CH, Yeung TC, Sweet DD, Doyle‐Waters MM, Schulzer M. The effect of a bolus dose of etomidate on cortisol levels, mortality, and health services utilization: a systematic review. Annals of Emergency Medicine 2010;56:105‐13. [PUBMED: 20346542] - PubMed
Lloyd 2013 [pers comm]
-
- Lloyd J. Inquiry Response for Hospira US2013‐11982 [personal communication]. E‐mail to: E Bruder 10 July, 2013.
Marik 2008
-
- Marik PE, Pastores SM, Annane D, Meduri GU, Sprung CL, Arlt W, et al. American College of Critical Care Medicine. Recommendations for the diagnosis and management of corticosteroid insufficiency in critically ill adult patients: consensus statements from an international task force by the American College of Critical Care Medicine. Critical Care Medicine 2008;36(6):1937‐49. [MEDLINE: ] - PubMed
RevMan 5.2 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roquilly 2011
-
- Roquilly A, Mahe PJ, Seguin P, Guitton C, Floch H, Tellier AC, et al. Hydrocortisone therapy for patients with multiple trauma: the randomized controlled HYPOLYTE study. JAMA 2011;305(12):1201‐9. [PUBMED: 21427372] - PubMed
Sivilotti 2003
-
- Sivilotti MLA, Filbin MR, Murray HE, Slasor P, Walls RM, NEAR Investigators. Does the sedative agent facilitate emergency rapid sequence intubation?. Academic Emergency Medicine 2003;10(6):612‐20. [MEDLINE: ] - PubMed
Sprung 2008
-
- Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. New England Journal of Medicine 2008;8:111‐24. [MEDLINE: ] - PubMed
Stoelting 2006
-
- Stoelting R, Hiller S. Pharmacology & Physiology in Anesthetic Practice. Fourth. Philadelphia: Lippincott Williams & Wilkins, 2006.
Truven 2013
-
- Truven Health Ac. Micromedex 2.0 SIU Conversion Calculator. http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexp... Accessed July, 2013.
Wong 1974
-
- Wong DHW, Jenkins LC. Experimental study of mechanism of action of ketamine on central nervous system. Canadian Anaesthetists Society Journal 1974;21(1):57‐67. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

