Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging
- PMID: 25556536
- PMCID: PMC4329416
- DOI: 10.1111/nan.12213
Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging
Erratum in
- Neuropathol Appl Neurobiol. 2015 Jun;41(4):571
-
Corrigendum.Neuropathol Appl Neurobiol. 2015 Jun;41(4):571. doi: 10.1111/nan.12240. Neuropathol Appl Neurobiol. 2015. PMID: 27210617 Free PMC article. No abstract available.
Abstract
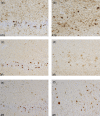
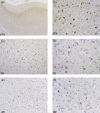
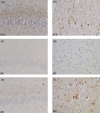
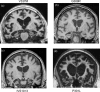
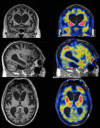
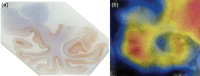
Hereditary frontotemporal dementia associated with mutations in the microtubule-associated protein tau gene (MAPT) is a protean disorder. Three neuropathologic subtypes can be recognized, based on the presence of inclusions made of tau isoforms with three and four repeats, predominantly three repeats and mostly four repeats. This is relevant for establishing a correlation between structural magnetic resonance imaging and positron emission tomography using tracers specific for aggregated tau. Longitudinal studies will be essential to determine the evolution of anatomical alterations from the asymptomatic stage to the various phases of disease following the onset of symptoms.
Keywords: FTDP-17 MAPT; Pick body; [F18]-T807; neurofibrillary tangle; tau; tau aggregation.
© 2014 The Authors. Neuropathology and Applied Neurobiology published by John Wiley & Sons Ltd on behalf of British Neuropathological Society.
Figures



Similar articles
-
Frontotemporal dementia with the V337M MAPT mutation: Tau-PET and pathology correlations.Neurology. 2017 Feb 21;88(8):758-766. doi: 10.1212/WNL.0000000000003636. Epub 2017 Jan 27. Neurology. 2017. PMID: 28130473 Free PMC article.
-
Tau PET Imaging with [18F]PM-PBB3 in Frontotemporal Dementia with MAPT Mutation.J Alzheimers Dis. 2020;76(1):149-157. doi: 10.3233/JAD-200287. J Alzheimers Dis. 2020. PMID: 32444551
-
Closing the tau loop: the missing tau mutation.Brain. 2015 Oct;138(Pt 10):3100-9. doi: 10.1093/brain/awv234. Epub 2015 Aug 21. Brain. 2015. PMID: 26297556 Free PMC article.
-
[The genetics of dementias. Part 1: Molecular basis of frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17)].Postepy Hig Med Dosw (Online). 2009 Jun 15;63:278-86. Postepy Hig Med Dosw (Online). 2009. PMID: 19535823 Review. Polish.
-
Genetics of Frontotemporal Dementia.Curr Neurol Neurosci Rep. 2016 Dec;16(12):107. doi: 10.1007/s11910-016-0707-9. Curr Neurol Neurosci Rep. 2016. PMID: 27878525 Review.
Cited by
-
Isoform-specific patterns of tau burden and neuronal degeneration in MAPT-associated frontotemporal lobar degeneration.Acta Neuropathol. 2022 Dec;144(6):1065-1084. doi: 10.1007/s00401-022-02487-4. Epub 2022 Sep 6. Acta Neuropathol. 2022. PMID: 36066634 Free PMC article.
-
Agitation and Dementia: Prevention and Treatment Strategies in Acute and Chronic Conditions.Front Neurol. 2021 Apr 16;12:644317. doi: 10.3389/fneur.2021.644317. eCollection 2021. Front Neurol. 2021. PMID: 33935943 Free PMC article. Review.
-
Increased 4R-Tau Induces Pathological Changes in a Human-Tau Mouse Model.Neuron. 2016 Jun 1;90(5):941-7. doi: 10.1016/j.neuron.2016.04.042. Epub 2016 May 19. Neuron. 2016. PMID: 27210553 Free PMC article.
-
Tau Isoforms: Gaining Insight into MAPT Alternative Splicing.Int J Mol Sci. 2022 Dec 6;23(23):15383. doi: 10.3390/ijms232315383. Int J Mol Sci. 2022. PMID: 36499709 Free PMC article. Review.
-
MAPT p.V363I mutation: A rare cause of corticobasal degeneration.Neurol Genet. 2019 Jun 25;5(4):e347. doi: 10.1212/NXG.0000000000000347. eCollection 2019 Aug. Neurol Genet. 2019. PMID: 31404212 Free PMC article.
References
-
- Vermaart W. Over de ziekte van pick. Nederl Tijdschr Geneesk. 1930;74:13.
-
- Schenk VW. Re-examination of a family with Pick's disease. Ann Hum Genet. 1959;23:325–333. - PubMed
-
- Groen JJ, Endtz LJ. Hereditary Pick's disease: second re-examination of the large family and discussion of other hereditary cases, with particular reference to electroencephalography, a computerized tomography. Brain. 1982;105(Pt 3):443–459. - PubMed
-
- Heston LL. The clinical genetics of Pick's disease. Acta Psychiatr Scand. 1978;57:202–206. - PubMed
-
- Foster NL, Wilhelmsen K, Sima AA, Jones MZ, D'Amato CJ, Gilman S. Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference participants. Ann Neurol. 1997;41:706–715. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

