SMAD4 loss triggers the phenotypic changes of pancreatic ductal adenocarcinoma cells
- PMID: 24625091
- PMCID: PMC4007528
- DOI: 10.1186/1471-2407-14-181
SMAD4 loss triggers the phenotypic changes of pancreatic ductal adenocarcinoma cells
Abstract
Background: SMAD4 is a gastrointestinal malignancy-specific tumor suppressor gene found mutated in one third of colorectal cancer specimens and half of pancreatic tumors. SMAD4 inactivation by allelic deletion or intragenic mutation mainly occurs in the late stage of human pancreatic ductal adenocarcinoma (PDAC). Various studies have proposed potential SMAD4-mediated anti-tumor effects in human malignancy; however, the relevance of SMAD4 in the PDAC molecular phenotype has not yet been fully characterized.
Methods: The AsPC-1, CFPAC-1 and PANC-1 human PDAC cell lines were used. The restoration or knockdown of SMAD4 expression in PDAC cells were confirmed by western blotting, luciferase reporter and immunofluorescence assays. In vitro cell proliferation, xenograft, wound healing, quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), Western blotting, and immunohistochemistry analysis were conducted using PDAC cells in which SMAD4 was either overexpressed or knocked down.
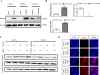
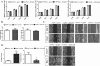
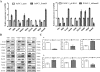
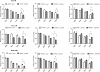
Results: Here, we report that re-expression of SMAD4 in SMAD4-null PDAC cells does not affect tumor cell growth in vitro or in vivo, but significantly enhances cells migration in vitro. SMAD4 restoration transcriptionally activates the TGF-β1/Nestin pathway and induces expression of several transcriptional factors. In contrast, SMAD4 loss in PDAC leads to increased expression of E-cadherin, vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR) and CD133. Furthermore, SMAD4 loss causes alterations to multiple kinase pathways (particularly the phosphorylated ERK/p38/Akt pathways), and increases chemoresistance in vitro. Finally, PDAC cells with intact SMAD4 are more sensitive to TGF-β1 inhibitor treatment to reduced cell migration; PDAC cells lacking SMAD4 showed decreased cell motility in response to EGFR inhibitor treatment.
Conclusions: This study revealed the molecular basis for SMAD4-dependent differences in PDAC with the aim of identifying the subset of patients likely to respond to therapies targeting the TGF-β or EGFR signaling pathways and of identifying potential therapeutic interventions for PDAC patients with SMAD4 defects.
Figures



Similar articles
-
Stem cell marker nestin is critical for TGF-β1-mediated tumor progression in pancreatic cancer.Mol Cancer Res. 2013 Jul;11(7):768-79. doi: 10.1158/1541-7786.MCR-12-0511. Epub 2013 Apr 3. Mol Cancer Res. 2013. PMID: 23552743
-
Down-regulation of microRNA-494 via loss of SMAD4 increases FOXM1 and β-catenin signaling in pancreatic ductal adenocarcinoma cells.Gastroenterology. 2014 Aug;147(2):485-97.e18. doi: 10.1053/j.gastro.2014.04.048. Epub 2014 May 20. Gastroenterology. 2014. PMID: 24859161
-
Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer.Genes Dev. 2006 Nov 15;20(22):3130-46. doi: 10.1101/gad.1478706. Genes Dev. 2006. PMID: 17114584 Free PMC article.
-
Localisation of PGK1 determines metabolic phenotype to balance metastasis and proliferation in patients with SMAD4-negative pancreatic cancer.Gut. 2020 May;69(5):888-900. doi: 10.1136/gutjnl-2018-317163. Epub 2019 Oct 14. Gut. 2020. PMID: 31611300 Review.
-
Clinicopathological significance of SMAD4 loss in pancreatic ductal adenocarcinomas: a systematic review and meta-analysis.Oncotarget. 2017 Mar 7;8(10):16704-16711. doi: 10.18632/oncotarget.14335. Oncotarget. 2017. PMID: 28053288 Free PMC article. Review.
Cited by
-
Tumor-Stromal Interactions in a Co-Culture Model of Human Pancreatic Adenocarcinoma Cells and Fibroblasts and Their Connection with Tumor Spread.Biomedicines. 2021 Mar 31;9(4):364. doi: 10.3390/biomedicines9040364. Biomedicines. 2021. PMID: 33807441 Free PMC article.
-
Spatiotemporal modulation of SMAD4 by HBx is required for cellular proliferation in hepatitis B-related liver cancer.Cell Oncol (Dordr). 2022 Aug;45(4):573-589. doi: 10.1007/s13402-022-00683-8. Epub 2022 Jun 18. Cell Oncol (Dordr). 2022. PMID: 35716259
-
Thymidine phosphorylase induction by ionizing radiation antagonizes 5-fluorouracil resistance in human ductal pancreatic adenocarcinoma.Radiat Environ Biophys. 2022 May;61(2):255-262. doi: 10.1007/s00411-022-00962-w. Epub 2022 Jan 27. Radiat Environ Biophys. 2022. PMID: 35084511 Free PMC article.
-
Pancreatic cancer stem cells.Am J Cancer Res. 2015 Feb 15;5(3):894-906. eCollection 2015. Am J Cancer Res. 2015. PMID: 26045976 Free PMC article. Review.
-
Identifying Drug Sensitivity Subnetworks with NETPHIX.iScience. 2020 Sep 29;23(10):101619. doi: 10.1016/j.isci.2020.101619. eCollection 2020 Oct 23. iScience. 2020. PMID: 33089107 Free PMC article.
References
-
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. - DOI - PubMed
-
- Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, Zhang H, Soon-Shiong P, Shi T, Rajeshkumar NV, Maitra A, Hidalgo M. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29(34):4548–54. doi: 10.1200/JCO.2011.36.5742. - DOI - PMC - PubMed
-
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. - DOI - PMC - PubMed
-
- Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001;7(4):251–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

