Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles
- PMID: 24512761
- PMCID: PMC4125554
- DOI: 10.1016/j.nano.2014.01.009
Protein corona composition does not accurately predict hematocompatibility of colloidal gold nanoparticles
Abstract
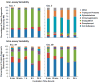
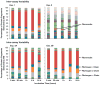
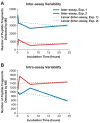
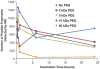
Proteins bound to nanoparticle surfaces are known to affect particle clearance by influencing immune cell uptake and distribution to the organs of the mononuclear phagocytic system. The composition of the protein corona has been described for several types of nanomaterials, but the role of the corona in nanoparticle biocompatibility is not well established. In this study we investigate the role of nanoparticle surface properties (PEGylation) and incubation times on the protein coronas of colloidal gold nanoparticles. While neither incubation time nor PEG molecular weight affected the specific proteins in the protein corona, the total amount of protein binding was governed by the molecular weight of PEG coating. Furthermore, the composition of the protein corona did not correlate with nanoparticle hematocompatibility. Specialized hematological tests should be used to deduce nanoparticle hematotoxicity. From the clinical editor: It is overall unclear how the protein corona associated with colloidal gold nanoparticles may influence hematotoxicity. This study warns that PEGylation itself may be insufficient, because composition of the protein corona does not directly correlate with nanoparticle hematocompatibility. The authors suggest that specialized hematological tests must be used to deduce nanoparticle hematotoxicity.
Keywords: Coagulation; Complement; Hematocompatibility; Nanoparticles; Protein corona.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Similar articles
-
Interaction of gold and silver nanoparticles with human plasma: Analysis of protein corona reveals specific binding patterns.Colloids Surf B Biointerfaces. 2017 Apr 1;152:317-325. doi: 10.1016/j.colsurfb.2017.01.037. Epub 2017 Jan 20. Colloids Surf B Biointerfaces. 2017. PMID: 28131092
-
Quantification of Lipid Corona Formation on Colloidal Nanoparticles from Lipid Vesicles.Anal Chem. 2018 Dec 18;90(24):14387-14394. doi: 10.1021/acs.analchem.8b03911. Epub 2018 Nov 20. Anal Chem. 2018. PMID: 30427176
-
Stealthiness and Hematocompatibility of Gold Nanoparticles with Pre-Formed Protein Corona.Langmuir. 2021 Apr 27;37(16):4913-4923. doi: 10.1021/acs.langmuir.1c00151. Epub 2021 Apr 16. Langmuir. 2021. PMID: 33861611
-
How Corona Formation Impacts Nanomaterials as Drug Carriers.Mol Pharm. 2020 Mar 2;17(3):725-737. doi: 10.1021/acs.molpharmaceut.9b01111. Epub 2020 Jan 24. Mol Pharm. 2020. PMID: 31939673 Review.
-
Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms.Curr Med Chem. 2018;25(35):4553-4586. doi: 10.2174/0929867325666180601101859. Curr Med Chem. 2018. PMID: 29852857 Free PMC article. Review.
Cited by
-
Designing Polyelectrolyte Microneedles Based on Borylated Poly(β-aminoester) Polymers To Enhance Transdermal pH-Controlled Delivery of Nucleic Acids.ACS Appl Polym Mater. 2024 Jul 24;6(15):8842-8855. doi: 10.1021/acsapm.4c00969. eCollection 2024 Aug 9. ACS Appl Polym Mater. 2024. PMID: 39144279 Free PMC article.
-
Gold Nanoparticles Contact with Cancer Cell: A Brief Update.Int J Mol Sci. 2022 Jul 12;23(14):7683. doi: 10.3390/ijms23147683. Int J Mol Sci. 2022. PMID: 35887030 Free PMC article. Review.
-
Engineered Exosomes-Based Photothermal Therapy with MRI/CT Imaging Guidance Enhances Anticancer Efficacy through Deep Tumor Nucleus Penetration.Pharmaceutics. 2021 Sep 30;13(10):1593. doi: 10.3390/pharmaceutics13101593. Pharmaceutics. 2021. PMID: 34683886 Free PMC article.
-
Impact of Protein Corona on the Biological Identity of Nanomedicine: Understanding the Fate of Nanomaterials in the Biological Milieu.Biomedicines. 2021 Oct 19;9(10):1496. doi: 10.3390/biomedicines9101496. Biomedicines. 2021. PMID: 34680613 Free PMC article. Review.
-
Current understanding of interactions between nanoparticles and the immune system.Toxicol Appl Pharmacol. 2016 May 15;299:78-89. doi: 10.1016/j.taap.2015.12.022. Epub 2015 Dec 29. Toxicol Appl Pharmacol. 2016. PMID: 26739622 Free PMC article. Review.
References
-
- Treuel L, Nienhaus GU. Nanoparticle interaction with plasma proteins as it relates to biodistribution. In: Dobrovolskaia MA, McNeil SE, editors. Handbook of immunological properties of engineered nanomaterials 1. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2013. pp. 151–64.
-
- Cedervall T, Lynch I, Foy M, Berggard T, Donnelly SC, Cagney G, et al. Detailed identification of plasma proteins adsorbed on copolymer nanoparticles. Angew Chem Int Ed Engl. 2007;46:5754–6. - PubMed
-
- Goppert TM, Muller RH. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: comparison of plasma protein adsorption patterns. J Drug Target. 2005;13:179–87. - PubMed
-
- Michaelis K, Hoffmann MM, Dreis S, Herbert E, Alyautdin RN, Michaelis M, et al. Covalent linkage of apolipoprotein e to albumin nanoparticles strongly enhances drug transport into the brain. J Pharmacol Exp Ther. 2006;317:1246–53. - PubMed
-
- Nagayama S, Ogawara K, Minato K, Fukuoka Y, Takakura Y, Hashida M, et al. Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor. Int J Pharm. 2007;329:192–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

