Trastuzumab-induced hepatotoxicity: a case report
- PMID: 24419371
- PMCID: PMC3683953
- DOI: 10.1159/000346844
Trastuzumab-induced hepatotoxicity: a case report
Abstract
Background: Trastuzumab is a humanized monoclonal antibody approved for the treatment of breast cancer with HER2 amplification and/or overexpression. There are only 2 prior cases of trastuzumab-related hepatotoxicity reported in the literature.
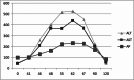
Case report: We report the case of a 60-year-old woman who was treated with trastuzumab for stage I invasive ductal carcinoma of the right breast. She successfully completed 6 months of therapy when an increase in liver transaminases was noted on routine examination. A full work-up for causes of acute and chronic liver disease was negative. After review of the patient's medication list, trastuzumab was thought to be the most likely culprit for the liver injury, based on timing of administration and rise in liver enzymes.
Hintergrund: Trastuzumab ist ein humanisierter monoklonaler Antikörper, der für die Behandlung von Brustkrebs mit HER2-Amplifikation und/oder -Überexpression zugelassen ist. Nur 2 frühere Fallberichte über eine Trastuzumab-assoziierte Hepatotoxizität sind in der Literatur zu finden.
Fallbericht: Wir berichten über den Fall einer 60-jährigen Frau, die aufgrund eines invasiven duktalen Karzinoms im Stadium I in der rechten Brust mit Trastuzumab behandelt wurde. Nach 6 Monaten erfolgreicher Therapie wurden bei ihr bei einer Routineuntersuchung erhöhte Lebertransaminasenspiegel festgestellt. Eine detaillierte Testauswertung hinsichtlich möglicher Auslöser einer akuten oder chronischen Lebererkrankung ergab ein negatives Resultat. Nach eingehender Prüfung der Medikationsliste der Patientin wurde aufgrund der zeitlichen Relation zwischen der Verabreichung und der Erhöhung der Leberenzymwerte Trastuzumab als die wahrscheinlichste Ursache für die Leberschädigung angenommen.
Keywords: Breast cancer; Hepatotoxicity; Liver toxicity; Trastuzumab.
Figures
Similar articles
-
Hepatotoxicity induced by trastuzumab used for breast cancer adjuvant therapy: a case report.J Med Case Rep. 2014 Dec 10;8:417. doi: 10.1186/1752-1947-8-417. J Med Case Rep. 2014. PMID: 25491149 Free PMC article.
-
Trastuzumab-induced hepatotoxicity.Ann Pharmacother. 2008 Oct;42(10):1497-501. doi: 10.1345/aph.1L217. Epub 2008 Sep 9. Ann Pharmacother. 2008. PMID: 18780811
-
Trastuzumab treatment of invasive breast ductal carcinoma induces severe edema: a case report.Transl Cancer Res. 2022 Nov;11(11):4185-4188. doi: 10.21037/tcr-22-1607. Transl Cancer Res. 2022. PMID: 36523319 Free PMC article.
-
Current and Emerging Therapies for HER2-Positive Women With Metastatic Breast Cancer.J Adv Pract Oncol. 2017 Mar;8(2):164-168. Epub 2017 Mar 1. J Adv Pract Oncol. 2017. PMID: 29900024 Free PMC article. Review.
-
Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer.Clin Ther. 1999 Feb;21(2):309-18. doi: 10.1016/S0149-2918(00)88288-0. Clin Ther. 1999. PMID: 10211534 Review.
Cited by
-
A phase I dose-escalation study of a biosimilar trastuzumab in Chinese metastasis breast cancer patients.Springerplus. 2015 Dec 22;4:803. doi: 10.1186/s40064-015-1603-5. eCollection 2015. Springerplus. 2015. PMID: 26702392 Free PMC article.
-
Management of hepatotoxicity of chemotherapy and targeted agents.Am J Cancer Res. 2021 Jul 15;11(7):3461-3474. eCollection 2021. Am J Cancer Res. 2021. PMID: 34354855 Free PMC article. Review.
-
Hepatotoxicity induced by trastuzumab used for breast cancer adjuvant therapy: a case report.J Med Case Rep. 2014 Dec 10;8:417. doi: 10.1186/1752-1947-8-417. J Med Case Rep. 2014. PMID: 25491149 Free PMC article.
-
Garlic Extract Alleviates Trastuzumab-Induced Hepatotoxicity in Rats Through Its Antioxidant, Anti-Inflammatory, and Antihyperlipidemic Effects.J Inflamm Res. 2021 Nov 27;14:6305-6316. doi: 10.2147/JIR.S339092. eCollection 2021. J Inflamm Res. 2021. PMID: 34866928 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. Erratum in CA Cancer J Clin 2011;61:133–134. - PubMed
-
- Daniele L, Sapino A. Anti-HER2 treatment and breast cancer: State of the art, recent patents, and new strategies. Recent Pat Anticancer Drug Discov. 2009;4:9–18. - PubMed
-
- Albanell J, Codony J, Rovira A, Mellado B, Gascon P. Mechanism of action of anti-HER2 monoclonal antibodies: Scientific update on trastuzumab and 2C4. Adv Exp Med Biol. 2003;532:253–268. - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Herceptin Adjuvant Trial Study T: Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

