Mutations in BCAP31 cause a severe X-linked phenotype with deafness, dystonia, and central hypomyelination and disorganize the Golgi apparatus
- PMID: 24011989
- PMCID: PMC3769969
- DOI: 10.1016/j.ajhg.2013.07.023
Mutations in BCAP31 cause a severe X-linked phenotype with deafness, dystonia, and central hypomyelination and disorganize the Golgi apparatus
Abstract
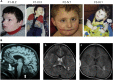
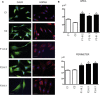
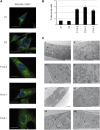
BAP31 is one of the most abundant endoplasmic reticulum (ER) membrane proteins. It is a chaperone protein involved in several pathways, including ER-associated degradation, export of ER proteins to the Golgi apparatus, and programmed cell death. BAP31 is encoded by BCAP31, located in human Xq28 and highly expressed in neurons. We identified loss-of-function mutations in BCAP31 in seven individuals from three families. These persons suffered from motor and intellectual disabilities, dystonia, sensorineural deafness, and white-matter changes, which together define an X-linked syndrome. In the primary fibroblasts of affected individuals, we found that BCAP31 deficiency altered ER morphology and caused a disorganization of the Golgi apparatus in a significant proportion of cells. Contrary to what has been described with transient-RNA-interference experiments, we demonstrate that constitutive BCAP31 deficiency does not activate the unfolded protein response or cell-death effectors. Rather, our data demonstrate that the lack of BAP31 disturbs ER metabolism and impacts the Golgi apparatus, highlighting an important role for BAP31 in ER-to-Golgi crosstalk. These findings provide a molecular basis for a Mendelian syndrome and link intracellular protein trafficking to severe congenital brain dysfunction and deafness.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Further delineation of BCAP31-linked intellectual disability: description of 17 new families with LoF and missense variants.Eur J Hum Genet. 2021 Sep;29(9):1405-1417. doi: 10.1038/s41431-021-00821-0. Epub 2021 Feb 18. Eur J Hum Genet. 2021. PMID: 33603160 Free PMC article.
-
BAP31: Physiological functions and roles in disease.Biochimie. 2021 Jul;186:105-129. doi: 10.1016/j.biochi.2021.04.008. Epub 2021 Apr 28. Biochimie. 2021. PMID: 33930507 Review.
-
Possible mitochondrial dysfunction in a patient with deafness, dystonia, and cerebral hypomyelination (DDCH) due to BCAP31 Mutation.Mol Genet Genomic Med. 2020 Mar;8(3):e1129. doi: 10.1002/mgg3.1129. Epub 2020 Jan 17. Mol Genet Genomic Med. 2020. PMID: 31953925 Free PMC article.
-
Schimke XLID syndrome results from a deletion in BCAP31.Am J Med Genet A. 2020 Sep;182(9):2168-2174. doi: 10.1002/ajmg.a.61755. Epub 2020 Jul 18. Am J Med Genet A. 2020. PMID: 32681719
-
Dystonia with and without deafness is caused by TIMM8A mutation.Adv Neurol. 2004;94:147-54. Adv Neurol. 2004. PMID: 14509668 Review. No abstract available.
Cited by
-
Oligodendrocyte and Extracellular Matrix Contributions to Central Nervous System Motor Function: Implications for Dystonia.Mov Disord. 2022 Mar;37(3):456-463. doi: 10.1002/mds.28892. Epub 2022 Jan 6. Mov Disord. 2022. PMID: 34989453 Free PMC article. Review.
-
Single paternal dexamethasone challenge programs offspring metabolism and reveals multiple candidates in RNA-mediated inheritance.iScience. 2021 Jul 16;24(8):102870. doi: 10.1016/j.isci.2021.102870. eCollection 2021 Aug 20. iScience. 2021. PMID: 34386731 Free PMC article.
-
BAP31 regulates mitochondrial function via interaction with Tom40 within ER-mitochondria contact sites.Sci Adv. 2019 Jun 12;5(6):eaaw1386. doi: 10.1126/sciadv.aaw1386. eCollection 2019 Jun. Sci Adv. 2019. PMID: 31206022 Free PMC article.
-
Hepatocyte-Specific Deficiency of BAP31 Amplified Acetaminophen-Induced Hepatotoxicity via Attenuating Nrf2 Signaling Activation in Mice.Int J Mol Sci. 2021 Oct 5;22(19):10788. doi: 10.3390/ijms221910788. Int J Mol Sci. 2021. PMID: 34639126 Free PMC article.
-
Interstitial lung disease and pancreatic exocrine insufficiency in CADDS: Phenotypic expansion and literature review.JIMD Rep. 2023 Aug 19;64(5):337-345. doi: 10.1002/jmd2.12390. eCollection 2023 Sep. JIMD Rep. 2023. PMID: 37701323 Free PMC article.
References
-
- Frédéric M.Y., Lalande M., Boileau C., Hamroun D., Claustres M., Béroud C., Collod-Béroud G. UMD-predictor, a new prediction tool for nucleotide substitution pathogenicity — application to four genes: FBN1, FBN2, TGFBR1, and TGFBR2. Hum. Mutat. 2009;30:952–959. - PubMed
-
- Bell A.W., Ward M.A., Blackstock W.P., Freeman H.N., Choudhary J.S., Lewis A.P., Chotai D., Fazel A., Gushue J.N., Paiement J. Proteomics characterization of abundant Golgi membrane proteins. J. Biol. Chem. 2001;276:5152–5165. - PubMed
-
- Paquet M.E., Cohen-Doyle M., Shore G.C., Williams D.B. Bap29/31 influences the intracellular traffic of MHC class I molecules. J. Immunol. 2004;172:7548–7555. - PubMed
-
- Wakana Y., Takai S., Nakajima K., Tani K., Yamamoto A., Watson P., Stephens D.J., Hauri H.P., Tagaya M. Bap31 is an itinerant protein that moves between the peripheral endoplasmic reticulum (ER) and a juxtanuclear compartment related to ER-associated Degradation. Mol. Biol. Cell. 2008;19:1825–1836. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

