Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome
- PMID: 24008845
- PMCID: PMC3807221
- DOI: 10.1038/embor.2013.132
Cyclic-di-GMP and cyclic-di-AMP activate the NLRP3 inflammasome
Abstract
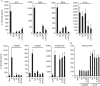
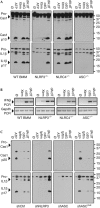
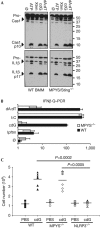
The cyclic dinucleotides 3'-5'diadenylate (c-diAMP) and 3'-5' diguanylate (c-diGMP) are important bacterial second messengers that have recently been shown to stimulate the secretion of type I Interferons (IFN-Is) through the c-diGMP-binding protein MPYS/STING. Here, we show that physiologically relevant levels of cyclic dinucleotides also stimulate a robust secretion of IL-1β through the NLRP3 inflammasome. Intriguingly, this response is independent of MPYS/STING. Consistent with most NLRP3 inflammasome activators, the response to c-diGMP is dependent on the mobilization of potassium and calcium ions. However, in contrast to other NLRP3 inflammasome activators, this response is not associated with significant changes in mitochondrial potential or the generation of mitochondrial reactive oxygen species. Thus, cyclic dinucleotides activate the NLRP3 inflammasome through a unique pathway that could have evolved to detect pervasive bacterial pathogen-associated molecular patterns associated with intracellular infections.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
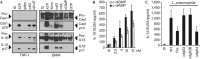
Similar articles
-
The anti-tumorigenic mushroom Agaricus blazei Murill enhances IL-1β production and activates the NLRP3 inflammasome in human macrophages.PLoS One. 2012;7(7):e41383. doi: 10.1371/journal.pone.0041383. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844468 Free PMC article.
-
Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1β secretion in association with alterations in cellular redox and energy status.Free Radic Biol Med. 2013 Jul;60:233-45. doi: 10.1016/j.freeradbiomed.2013.01.025. Epub 2013 Jan 29. Free Radic Biol Med. 2013. PMID: 23376234 Free PMC article.
-
Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome.PLoS Pathog. 2012;8(8):e1002857. doi: 10.1371/journal.ppat.1002857. Epub 2012 Aug 9. PLoS Pathog. 2012. PMID: 22916014 Free PMC article.
-
Signal Transduction of Streptococci by Cyclic Dinucleotide Second Messengers.Curr Issues Mol Biol. 2019;32:87-122. doi: 10.21775/cimb.032.087. Epub 2019 Jun 5. Curr Issues Mol Biol. 2019. PMID: 31166170 Review.
-
Cyclic nucleotides, gut physiology and inflammation.FEBS J. 2020 May;287(10):1970-1981. doi: 10.1111/febs.15198. Epub 2020 Jan 14. FEBS J. 2020. PMID: 31889413 Free PMC article. Review.
Cited by
-
cGAS deficiency enhances inflammasome activation in macrophages and inflammatory pathology in pristane-induced lupus.Front Immunol. 2022 Dec 16;13:1010764. doi: 10.3389/fimmu.2022.1010764. eCollection 2022. Front Immunol. 2022. PMID: 36591278 Free PMC article.
-
Inflammasomes in Myeloid Cells: Warriors Within.Microbiol Spectr. 2017 Jan;5(1):10.1128/microbiolspec.mchd-0049-2016. doi: 10.1128/microbiolspec.MCHD-0049-2016. Microbiol Spectr. 2017. PMID: 28102121 Free PMC article. Review.
-
The NLRP3 inflammasome: molecular activation and regulation to therapeutics.Nat Rev Immunol. 2019 Aug;19(8):477-489. doi: 10.1038/s41577-019-0165-0. Nat Rev Immunol. 2019. PMID: 31036962 Free PMC article. Review.
-
Intratumoral STING Activation with T-cell Checkpoint Modulation Generates Systemic Antitumor Immunity.Cancer Immunol Res. 2017 Aug;5(8):676-684. doi: 10.1158/2326-6066.CIR-17-0049. Epub 2017 Jul 3. Cancer Immunol Res. 2017. PMID: 28674082 Free PMC article.
-
The role of bacterial cyclic di-adenosine monophosphate in the host immune response.Front Microbiol. 2022 Aug 29;13:958133. doi: 10.3389/fmicb.2022.958133. eCollection 2022. Front Microbiol. 2022. PMID: 36106081 Free PMC article. Review.
References
-
- Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34 - PubMed
-
- Magalhaes JG, Sorbara MT, Girardin SE, Philpott DJ (2011) What is new with Nods? Curr Opin Immunol 23: 29–34 - PubMed
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481: 278–286 - PubMed
-
- Lamkanfi M, Dixit VM (2012) Inflammasomes and their roles in health and disease. Ann Rev Cell Dev Biol 28: 137–161 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI096088/AI/NIAID NIH HHS/United States
- T32AI074491/AI/NIAID NIH HHS/United States
- K01 DK092355/DK/NIDDK NIH HHS/United States
- R01 AR061491/AR/NIAMS NIH HHS/United States
- R01 AI062739/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- R01 AI058211/AI/NIAID NIH HHS/United States
- AI057157/AI/NIAID NIH HHS/United States
- AI058211/AI/NIAID NIH HHS/United States
- AR061491/AR/NIAMS NIH HHS/United States
- R37-AI029564/AI/NIAID NIH HHS/United States
- T32 AI074491/AI/NIAID NIH HHS/United States
- AI062739/AI/NIAID NIH HHS/United States
- R37 AI029564/AI/NIAID NIH HHS/United States
- R21 AI096088/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

