Salirasib sensitizes hepatocarcinoma cells to TRAIL-induced apoptosis through DR5 and survivin-dependent mechanisms
- PMID: 23348585
- PMCID: PMC3563988
- DOI: 10.1038/cddis.2012.200
Salirasib sensitizes hepatocarcinoma cells to TRAIL-induced apoptosis through DR5 and survivin-dependent mechanisms
Abstract
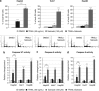
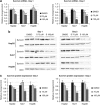
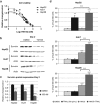
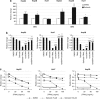
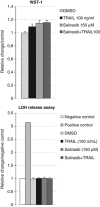
Ras activation is a frequent event in human hepatocarcinoma that may contribute to resistance towards apoptosis. Salirasib is a ras and mTOR inhibitor that induces a pro-apoptotic phenotype in human hepatocarcinoma cell lines. In this work, we evaluate whether salirasib sensitizes those cells to TRAIL-induced apoptosis. Cell viability, cell death and apoptosis were evaluated in vitro in HepG2, Hep3B and Huh7 cells treated with DMSO, salirasib and YM155 (a survivin inhibitor), alone or in combination with recombinant TRAIL. Our results show that pretreatment with salirasib sensitized human hepatocarcinoma cell lines, but not normal human hepatocytes, to TRAIL-induced apoptosis. Indeed, FACS analysis showed that 25 (Huh7) to 50 (HepG2 and Hep3B) percent of the cells treated with both drugs were apoptotic. This occurred through activation of the extrinsic and the intrinsic pathways, as evidenced by a marked increase in caspase 3/7 (five to ninefold), caspase 8 (four to sevenfold) and caspase 9 (eight to 12-fold) activities in cells treated with salirasib and TRAIL compared with control. Survivin inhibition had an important role in this process and was sufficient to sensitize hepatocarcinoma cells to apoptosis. Furthermore, TRAIL-induced apoptosis in HCC cells pretreated with salirasib was dependent on activation of death receptor (DR) 5. In conclusion, salirasib sensitizes hepatocarcinoma cells to TRAIL-induced apoptosis by a mechanism involving the DR5 receptor and survivin inhibition. These results in human hepatocarcinoma cell lines and primary hepatocytes provide a rationale for testing the combination of salirasib and TRAIL agonists in human hepatocarcinoma.
Figures

Similar articles
-
Salirasib inhibits the growth of hepatocarcinoma cell lines in vitro and tumor growth in vivo through ras and mTOR inhibition.Mol Cancer. 2010 Sep 22;9:256. doi: 10.1186/1476-4598-9-256. Mol Cancer. 2010. PMID: 20860815 Free PMC article.
-
Low-dose 5-fluorouracil sensitizes HepG2 cells to TRAIL through TRAIL receptor DR5 and survivin-dependent mechanisms.J Chemother. 2017 Jun;29(3):179-188. doi: 10.1080/1120009X.2016.1277048. Epub 2017 Jan 9. J Chemother. 2017. PMID: 28067150
-
TRAIL enhances apoptosis of human hepatocellular carcinoma cells sensitized by hepatitis C virus infection: therapeutic implications.PLoS One. 2014 Jun 13;9(6):e98171. doi: 10.1371/journal.pone.0098171. eCollection 2014. PLoS One. 2014. PMID: 24927176 Free PMC article.
-
Hep G2 Hepatocarcinoma Homogeneous Apoptosis Assay: Version 1.2.2011 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method GTA-14. 2011 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method GTA-14. PMID: 39012969 Free Books & Documents. Review.
-
HepG2 Hepatocarcinoma Apoptosis Assay: Version 1.1.2010 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method GTA-6. 2010 Apr. In: National Cancer Institute’s Nanotechnology Characterization Laboratory Assay Cascade Protocols [Internet]. Bethesda (MD): National Cancer Institute (US); 2005 May 1–. NCL Method GTA-6. PMID: 39012975 Free Books & Documents. Review.
Cited by
-
Multifunctionalized polyethyleneimine-based nanocarriers for gene and chemotherapeutic drug combination therapy through one-step assembly strategy.Int J Nanomedicine. 2017 Dec 6;12:8681-8698. doi: 10.2147/IJN.S142966. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29263663 Free PMC article.
-
Farnesylthiosalicylic acid sensitizes hepatocarcinoma cells to artemisinin derivatives.PLoS One. 2017 Feb 9;12(2):e0171840. doi: 10.1371/journal.pone.0171840. eCollection 2017. PLoS One. 2017. PMID: 28182780 Free PMC article.
-
Ras Signaling Inhibitors Attenuate Disease in Adjuvant-Induced Arthritis via Targeting Pathogenic Antigen-Specific Th17-Type Cells.Front Immunol. 2017 Jul 7;8:799. doi: 10.3389/fimmu.2017.00799. eCollection 2017. Front Immunol. 2017. PMID: 28736556 Free PMC article.
-
Phase I study of S-trans, trans-farnesylthiosalicylic acid (salirasib), a novel oral RAS inhibitor in patients with refractory hematologic malignancies.Clin Lymphoma Myeloma Leuk. 2015 Jul;15(7):433-438.e2. doi: 10.1016/j.clml.2015.02.018. Epub 2015 Feb 19. Clin Lymphoma Myeloma Leuk. 2015. PMID: 25795639 Free PMC article. Clinical Trial.
-
Identification and validation of Birc5 as a novel activated cell cycle program biomarker associated with infiltration of immunosuppressive myeloid-derived suppressor cells in hepatocellular carcinoma.Cancer Med. 2023 Aug;12(15):16370-16385. doi: 10.1002/cam4.6271. Epub 2023 Jun 16. Cancer Med. 2023. PMID: 37326143 Free PMC article.
References
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Schattenberg JM, Schuchmann M, Galle PR. Cell death and hepatocarcinogenesis: Dysregulation of apoptosis signaling pathways. J Gastroenterol Hepatol. 2011;26 (Suppl 1:213–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

