Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants
- PMID: 23035049
- PMCID: PMC3606011
- DOI: 10.1093/hmg/dds421
Determining consequences of retinal membrane guanylyl cyclase (RetGC1) deficiency in human Leber congenital amaurosis en route to therapy: residual cone-photoreceptor vision correlates with biochemical properties of the mutants
Abstract
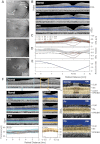
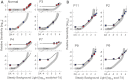
The GUCY2D gene encodes retinal membrane guanylyl cyclase (RetGC1), a key component of the phototransduction machinery in photoreceptors. Mutations in GUCY2D cause Leber congenital amaurosis type 1 (LCA1), an autosomal recessive human retinal blinding disease. The effects of RetGC1 deficiency on human rod and cone photoreceptor structure and function are currently unknown. To move LCA1 closer to clinical trials, we characterized a cohort of patients (ages 6 months-37 years) with GUCY2D mutations. In vivo analyses of retinal architecture indicated intact rod photoreceptors in all patients but abnormalities in foveal cones. By functional phenotype, there were patients with and those without detectable cone vision. Rod vision could be retained and did not correlate with the extent of cone vision or age. In patients without cone vision, rod vision functioned unsaturated under bright ambient illumination. In vitro analyses of the mutant alleles showed that in addition to the major truncation of the essential catalytic domain in RetGC1, some missense mutations in LCA1 patients result in a severe loss of function by inactivating its catalytic activity and/or ability to interact with the activator proteins, GCAPs. The differences in rod sensitivities among patients were not explained by the biochemical properties of the mutants. However, the RetGC1 mutant alleles with remaining biochemical activity in vitro were associated with retained cone vision in vivo. We postulate a relationship between the level of RetGC1 activity and the degree of cone vision abnormality, and argue for cone function being the efficacy outcome in clinical trials of gene augmentation therapy in LCA1.
Figures


Similar articles
-
GUCY2D mutations in retinal guanylyl cyclase 1 provide biochemical reasons for dominant cone-rod dystrophy but not for stationary night blindness.J Biol Chem. 2020 Dec 25;295(52):18301-18315. doi: 10.1074/jbc.RA120.015553. Epub 2020 Oct 27. J Biol Chem. 2020. PMID: 33109612 Free PMC article.
-
Gene Therapy Fully Restores Vision to the All-Cone Nrl(-/-) Gucy2e(-/-) Mouse Model of Leber Congenital Amaurosis-1.Hum Gene Ther. 2015 Sep;26(9):575-92. doi: 10.1089/hum.2015.053. Epub 2015 Aug 6. Hum Gene Ther. 2015. PMID: 26247368 Free PMC article.
-
AAV-mediated gene therapy in the guanylate cyclase (RetGC1/RetGC2) double knockout mouse model of Leber congenital amaurosis.Hum Gene Ther. 2013 Feb;24(2):189-202. doi: 10.1089/hum.2012.193. Hum Gene Ther. 2013. PMID: 23210611 Free PMC article.
-
A Mini-review: Animal Models of GUCY2D Leber Congenital Amaurosis (LCA1).Adv Exp Med Biol. 2016;854:253-8. doi: 10.1007/978-3-319-17121-0_34. Adv Exp Med Biol. 2016. PMID: 26427419 Review.
-
Insights gained from gene therapy in animal models of retGC1 deficiency.Front Mol Neurosci. 2014 May 14;7:43. doi: 10.3389/fnmol.2014.00043. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24860425 Free PMC article. Review.
Cited by
-
Durable vision improvement after a single treatment with antisense oligonucleotide sepofarsen: a case report.Nat Med. 2021 May;27(5):785-789. doi: 10.1038/s41591-021-01297-7. Epub 2021 Apr 1. Nat Med. 2021. PMID: 33795869 Free PMC article.
-
Transient pupillary light reflex in CEP290- or NPHP5-associated Leber congenital amaurosis: Latency as a potential outcome measure of cone function.Vision Res. 2020 Mar;168:53-63. doi: 10.1016/j.visres.2020.01.006. Epub 2020 Feb 20. Vision Res. 2020. PMID: 32088401 Free PMC article.
-
GUCY2D mutations in retinal guanylyl cyclase 1 provide biochemical reasons for dominant cone-rod dystrophy but not for stationary night blindness.J Biol Chem. 2020 Dec 25;295(52):18301-18315. doi: 10.1074/jbc.RA120.015553. Epub 2020 Oct 27. J Biol Chem. 2020. PMID: 33109612 Free PMC article.
-
Regulation of retinal membrane guanylyl cyclase (RetGC) by negative calcium feedback and RD3 protein.Pflugers Arch. 2021 Sep;473(9):1393-1410. doi: 10.1007/s00424-021-02523-4. Epub 2021 Feb 3. Pflugers Arch. 2021. PMID: 33537894 Free PMC article. Review.
-
The Clinical Findings, Pathogenic Variants, and Gene Therapy Qualifications Found in a Leber Congenital Amaurosis Phenotypic Spectrum Patient Cohort.Int J Mol Sci. 2024 Jan 19;25(2):1253. doi: 10.3390/ijms25021253. Int J Mol Sci. 2024. PMID: 38279252 Free PMC article.
References
-
- Ripps H. Light to sight: milestones in phototransduction. FASEB J. 2010;24:970–975. - PubMed
-
- Bramall A.N., Wright A.F., Jacobson S.G., McInnes R.R. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu. Rev. Neurosci. 2010;33:441–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

