An essential role of variant histone H3.3 for ectomesenchyme potential of the cranial neural crest
- PMID: 23028350
- PMCID: PMC3447937
- DOI: 10.1371/journal.pgen.1002938
An essential role of variant histone H3.3 for ectomesenchyme potential of the cranial neural crest
Abstract
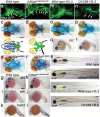
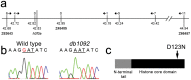
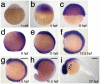
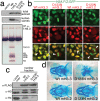
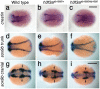
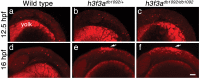
The neural crest (NC) is a vertebrate-specific cell population that exhibits remarkable multipotency. Although derived from the neural plate border (NPB) ectoderm, cranial NC (CNC) cells contribute not only to the peripheral nervous system but also to the ectomesenchymal precursors of the head skeleton. To date, the developmental basis for such broad potential has remained elusive. Here, we show that the replacement histone H3.3 is essential during early CNC development for these cells to generate ectomesenchyme and head pigment precursors. In a forward genetic screen in zebrafish, we identified a dominant D123N mutation in h3f3a, one of five zebrafish variant histone H3.3 genes, that eliminates the CNC-derived head skeleton and a subset of pigment cells yet leaves other CNC derivatives and trunk NC intact. Analyses of nucleosome assembly indicate that mutant D123N H3.3 interferes with H3.3 nucleosomal incorporation by forming aberrant H3 homodimers. Consistent with CNC defects arising from insufficient H3.3 incorporation into chromatin, supplying exogenous wild-type H3.3 rescues head skeletal development in mutants. Surprisingly, embryo-wide expression of dominant mutant H3.3 had little effect on embryonic development outside CNC, indicating an unexpectedly specific sensitivity of CNC to defects in H3.3 incorporation. Whereas previous studies had implicated H3.3 in large-scale histone replacement events that generate totipotency during germ line development, our work has revealed an additional role of H3.3 in the broad potential of the ectoderm-derived CNC, including the ability to make the mesoderm-like ectomesenchymal precursors of the head skeleton.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Reassessing the embryonic origin and potential of craniofacial ectomesenchyme.Semin Cell Dev Biol. 2023 Mar 30;138:45-53. doi: 10.1016/j.semcdb.2022.03.018. Epub 2022 Mar 21. Semin Cell Dev Biol. 2023. PMID: 35331627 Free PMC article. Review.
-
Bmps and id2a act upstream of Twist1 to restrict ectomesenchyme potential of the cranial neural crest.PLoS Genet. 2012;8(5):e1002710. doi: 10.1371/journal.pgen.1002710. Epub 2012 May 10. PLoS Genet. 2012. PMID: 22589745 Free PMC article.
-
Znf703 is a novel RA target in the neural plate border.Sci Rep. 2019 Jun 4;9(1):8275. doi: 10.1038/s41598-019-44722-1. Sci Rep. 2019. PMID: 31164691 Free PMC article.
-
The issue of the multipotency of the neural crest cells.Dev Biol. 2018 Dec 1;444 Suppl 1:S47-S59. doi: 10.1016/j.ydbio.2018.03.024. Epub 2018 Mar 31. Dev Biol. 2018. PMID: 29614271 Review.
-
BMP, Wnt and FGF signals are integrated through evolutionarily conserved enhancers to achieve robust expression of Pax3 and Zic genes at the zebrafish neural plate border.Development. 2012 Nov;139(22):4220-31. doi: 10.1242/dev.081497. Epub 2012 Oct 3. Development. 2012. PMID: 23034628 Free PMC article.
Cited by
-
Point mutations in an epigenetic factor lead to multiple types of bone tumors: role of H3.3 histone variant in bone development and disease.Bonekey Rep. 2015 Jul 1;4:715. doi: 10.1038/bonekey.2015.84. eCollection 2015. Bonekey Rep. 2015. PMID: 26157578 Free PMC article. Review.
-
Distinct chromatin signature of histone H3 variant H3.3 in human cells.Nucleus. 2014 Sep-Oct;5(5):449-61. doi: 10.4161/nucl.36229. Nucleus. 2014. PMID: 25482197 Free PMC article.
-
Histone variant H3.3 maintains a decondensed chromatin state essential for mouse preimplantation development.Development. 2013 Sep;140(17):3624-34. doi: 10.1242/dev.095513. Epub 2013 Jul 31. Development. 2013. PMID: 23903189 Free PMC article.
-
Reassessing the embryonic origin and potential of craniofacial ectomesenchyme.Semin Cell Dev Biol. 2023 Mar 30;138:45-53. doi: 10.1016/j.semcdb.2022.03.018. Epub 2022 Mar 21. Semin Cell Dev Biol. 2023. PMID: 35331627 Free PMC article. Review.
-
TFAP2 paralogs regulate midfacial development in part through a conserved ALX genetic pathway.Development. 2024 Jan 1;151(1):dev202095. doi: 10.1242/dev.202095. Epub 2024 Jan 2. Development. 2024. PMID: 38063857 Free PMC article.
References
-
- Gans C, Northcutt RG (1983) Neural Crest and the Origin of Vertebrates: A New Head. Science 220: 268–273. - PubMed
-
- Baroffio A, Dupin E, Le Douarin NM (1991) Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development 112: 301–305. - PubMed
-
- Bronner-Fraser M, Fraser SE (1988) Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature 335: 161–164. - PubMed
-
- Platt JB (1893) Ectodermic origin of the cartilages of the head. Anat Anz 8: 506–509.
-
- Schilling TF, Kimmel CB (1994) Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120: 483–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

