Trastuzumab emtansine for HER2-positive advanced breast cancer
- PMID: 23020162
- PMCID: PMC5125250
- DOI: 10.1056/NEJMoa1209124
Trastuzumab emtansine for HER2-positive advanced breast cancer
Erratum in
- N Engl J Med. 2013 Jun 20;368(25):2442
Abstract
Background: Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate incorporating the human epidermal growth factor receptor 2 (HER2)-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1. The antibody and the cytotoxic agent are conjugated by means of a stable linker.
Methods: We randomly assigned patients with HER2-positive advanced breast cancer, who had previously been treated with trastuzumab and a taxane, to T-DM1 or lapatinib plus capecitabine. The primary end points were progression-free survival (as assessed by independent review), overall survival, and safety. Secondary end points included progression-free survival (investigator-assessed), the objective response rate, and the time to symptom progression. Two interim analyses of overall survival were conducted.
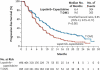
Results: Among 991 randomly assigned patients, median progression-free survival as assessed by independent review was 9.6 months with T-DM1 versus 6.4 months with lapatinib plus capecitabine (hazard ratio for progression or death from any cause, 0.65; 95% confidence interval [CI], 0.55 to 0.77; P<0.001), and median overall survival at the second interim analysis crossed the stopping boundary for efficacy (30.9 months vs. 25.1 months; hazard ratio for death from any cause, 0.68; 95% CI, 0.55 to 0.85; P<0.001). The objective response rate was higher with T-DM1 (43.6%, vs. 30.8% with lapatinib plus capecitabine; P<0.001); results for all additional secondary end points favored T-DM1. Rates of grade 3 or 4 adverse events were higher with lapatinib plus capecitabine than with T-DM1 (57% vs. 41%). The incidences of thrombocytopenia and increased serum aminotransferase levels were higher with T-DM1, whereas the incidences of diarrhea, nausea, vomiting, and palmar-plantar erythrodysesthesia were higher with lapatinib plus capecitabine.
Conclusions: T-DM1 significantly prolonged progression-free and overall survival with less toxicity than lapatinib plus capecitabine in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane. (Funded by F. Hoffmann-La Roche/Genentech; EMILIA ClinicalTrials.gov number, NCT00829166.).
Figures

Comment in
-
The promise of antibody-drug conjugates.N Engl J Med. 2012 Nov 8;367(19):1847-8. doi: 10.1056/NEJMe1211736. N Engl J Med. 2012. PMID: 23134386 No abstract available.
Similar articles
-
Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial.Lancet Oncol. 2017 Jun;18(6):732-742. doi: 10.1016/S1470-2045(17)30312-1. Epub 2017 May 16. Lancet Oncol. 2017. PMID: 28526536 Free PMC article. Clinical Trial.
-
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA.Ann Oncol. 2015 Jan;26(1):113-119. doi: 10.1093/annonc/mdu486. Epub 2014 Oct 29. Ann Oncol. 2015. PMID: 25355722 Free PMC article. Clinical Trial.
-
Patient-reported outcomes from EMILIA, a randomized phase 3 study of trastuzumab emtansine (T-DM1) versus capecitabine and lapatinib in human epidermal growth factor receptor 2-positive locally advanced or metastatic breast cancer.Cancer. 2014 Mar 1;120(5):642-51. doi: 10.1002/cncr.28465. Epub 2013 Nov 12. Cancer. 2014. PMID: 24222194 Clinical Trial.
-
Ado-trastuzumab emtansine: a HER2-positive targeted antibody-drug conjugate.Ann Pharmacother. 2014 Nov;48(11):1484-93. doi: 10.1177/1060028014545354. Epub 2014 Jul 31. Ann Pharmacother. 2014. PMID: 25082874 Review.
-
Trastuzumab Emtansine for Treating HER2-Positive, Unresectable, Locally Advanced or Metastatic Breast Cancer After Treatment with Trastuzumab and a Taxane: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2016 Jul;34(7):673-80. doi: 10.1007/s40273-016-0386-z. Pharmacoeconomics. 2016. PMID: 26892972 Review.
Cited by
-
TP53-positive clones are responsible for drug-tolerant persister and recurrence of HER2-positive breast cancer.Breast Cancer Res Treat. 2022 Nov;196(2):255-266. doi: 10.1007/s10549-022-06731-z. Epub 2022 Sep 10. Breast Cancer Res Treat. 2022. PMID: 36087189
-
Breast cancer in 2012: New drugs, new knowledge, new targets.Nat Rev Clin Oncol. 2013 Feb;10(2):75-6. doi: 10.1038/nrclinonc.2012.236. Epub 2013 Jan 8. Nat Rev Clin Oncol. 2013. PMID: 23296111 Review. No abstract available.
-
Two engineered site-specific antibody-drug conjugates, HLmD4 and HLvM4, have potent therapeutic activity in two DLL4-positive tumour xenograft models.Am J Cancer Res. 2020 Aug 1;10(8):2387-2408. eCollection 2020. Am J Cancer Res. 2020. PMID: 32905508 Free PMC article.
-
Targeted Therapies in Non-Small Cell Lung Cancer-Beyond EGFR and ALK.Cancers (Basel). 2015 May 26;7(2):930-49. doi: 10.3390/cancers7020816. Cancers (Basel). 2015. PMID: 26018876 Free PMC article. Review.
-
Prostate-Specific Membrane Antigen-Directed Therapy for Metastatic Castration-Resistant Prostate Cancer.Cancer J. 2016 Sep/Oct;22(5):347-352. doi: 10.1097/PPO.0000000000000221. Cancer J. 2016. PMID: 27749329 Free PMC article. Review.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for meta-static breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 Study Group. J Clin Oncol. 2005;23:4265–74. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
