Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome
- PMID: 22634751
- PMCID: PMC3386373
- DOI: 10.1038/ng.2275
Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome
Abstract
IMAGe syndrome (intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia congenita and genital anomalies) is an undergrowth developmental disorder with life-threatening consequences. An identity-by-descent analysis in a family with IMAGe syndrome identified a 17.2-Mb locus on chromosome 11p15 that segregated in the affected family members. Targeted exon array capture of the disease locus, followed by high-throughput genomic sequencing and validation by dideoxy sequencing, identified missense mutations in the imprinted gene CDKN1C (also known as P57KIP2) in two familial and four unrelated patients. A familial analysis showed an imprinted mode of inheritance in which only maternal transmission of the mutation resulted in IMAGe syndrome. CDKN1C inhibits cell-cycle progression, and we found that targeted expression of IMAGe-associated CDKN1C mutations in Drosophila caused severe eye growth defects compared to wild-type CDKN1C, suggesting a gain-of-function mechanism. All IMAGe-associated mutations clustered in the PCNA-binding domain of CDKN1C and resulted in loss of PCNA binding, distinguishing them from the mutations of CDKN1C that cause Beckwith-Wiedemann syndrome, an overgrowth syndrome.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
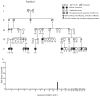



Comment in
-
Gain of function in CDKN1C.Nat Genet. 2012 Jun 27;44(7):737-8. doi: 10.1038/ng.2336. Nat Genet. 2012. PMID: 22735584 No abstract available.
Similar articles
-
Gain of function in CDKN1C.Nat Genet. 2012 Jun 27;44(7):737-8. doi: 10.1038/ng.2336. Nat Genet. 2012. PMID: 22735584 No abstract available.
-
A novel variant in CDKN1C is associated with intrauterine growth restriction, short stature, and early-adulthood-onset diabetes.J Clin Endocrinol Metab. 2014 Oct;99(10):E2117-22. doi: 10.1210/jc.2014-1949. Epub 2014 Jul 24. J Clin Endocrinol Metab. 2014. PMID: 25057881 Free PMC article.
-
CDKN1C mutations: two sides of the same coin.Trends Mol Med. 2014 Nov;20(11):614-22. doi: 10.1016/j.molmed.2014.09.001. Epub 2014 Sep 25. Trends Mol Med. 2014. PMID: 25262539 Review.
-
CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome.J Med Genet. 2013 Dec;50(12):823-30. doi: 10.1136/jmedgenet-2013-101691. Epub 2013 Sep 24. J Med Genet. 2013. PMID: 24065356
-
Analysis of CDKN1C in fetal growth restriction and pregnancy loss.F1000Res. 2019 Jan 23;8:90. doi: 10.12688/f1000research.15016.2. eCollection 2019. F1000Res. 2019. PMID: 31497289 Free PMC article. Review.
Cited by
-
The role and interaction of imprinted genes in human fetal growth.Philos Trans R Soc Lond B Biol Sci. 2015 Mar 5;370(1663):20140074. doi: 10.1098/rstb.2014.0074. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25602077 Free PMC article. Review.
-
Genetic Analysis of Pediatric Primary Adrenal Insufficiency of Unknown Etiology: 25 Years' Experience in the UK.J Endocr Soc. 2021 May 11;5(8):bvab086. doi: 10.1210/jendso/bvab086. eCollection 2021 Aug 1. J Endocr Soc. 2021. PMID: 34258490 Free PMC article.
-
Advances in Skeletal Dysplasia Genetics.Annu Rev Genomics Hum Genet. 2015;16:199-227. doi: 10.1146/annurev-genom-090314-045904. Epub 2015 Apr 22. Annu Rev Genomics Hum Genet. 2015. PMID: 25939055 Free PMC article. Review.
-
New developments in Silver-Russell syndrome and implications for clinical practice.Epigenomics. 2016 Apr;8(4):563-80. doi: 10.2217/epi-2015-0010. Epub 2016 Apr 12. Epigenomics. 2016. PMID: 27066913 Free PMC article. Review.
-
Uniparental disomy as a cause of pediatric endocrine disorders.Clin Pediatr Endocrinol. 2018;27(3):113-121. doi: 10.1297/cpe.27.113. Epub 2018 Jul 31. Clin Pediatr Endocrinol. 2018. PMID: 30083028 Free PMC article.
References
-
- Vilain E, et al. IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab. 1999;84:4335–4340. - PubMed
-
- Bergada I, et al. Familial occurrence of the IMAGe association: additional clinical variants and a proposed mode of inheritance. J Clin Endocrinol Metab. 2005;90:3186–3190. - PubMed
-
- Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9:639–649. - PubMed
-
- Romanelli V, et al. CDKN1C (p57(Kip2)) analysis in Beckwith-Wiedemann syndrome (BWS) patients: Genotype-phenotype correlations, novel mutations, and polymorphisms. Am J Med Genet A. 2010;152A:1390–1397. - PubMed
-
- Hutz JE, et al. IMAGe association and congenital adrenal hypoplasia: no disease-causing mutations found in the ACD gene. Mol Genet Metab. 2006;88:66–70. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

