Loss of neuronal potassium/chloride cotransporter 3 (KCC3) is responsible for the degenerative phenotype in a conditional mouse model of hereditary motor and sensory neuropathy associated with agenesis of the corpus callosum
- PMID: 22423107
- PMCID: PMC6703451
- DOI: 10.1523/JNEUROSCI.3679-11.2012
Loss of neuronal potassium/chloride cotransporter 3 (KCC3) is responsible for the degenerative phenotype in a conditional mouse model of hereditary motor and sensory neuropathy associated with agenesis of the corpus callosum
Abstract
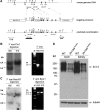
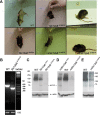
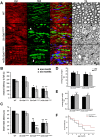
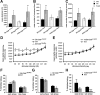
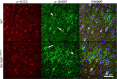
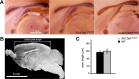
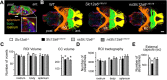
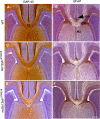
Disruption of the potassium/chloride cotransporter 3 (KCC3), encoded by the SLC12A6 gene, causes hereditary motor and sensory neuropathy associated with agenesis of the corpus callosum (HMSN/ACC), a neurodevelopmental and neurodegenerative disorder affecting both the peripheral nervous system and CNS. However, the precise role of KCC3 in the maintenance of ion homeostasis in the nervous system and the pathogenic mechanisms leading to HMSN/ACC remain unclear. We established two Slc12a6 Cre/LoxP transgenic mouse lines expressing C-terminal truncated KCC3 in either a neuron-specific or ubiquitous fashion. Our results suggest that neuronal KCC3 expression is crucial for axon volume control. We also demonstrate that the neuropathic features of HMSN/ACC are predominantly due to a neuronal KCC3 deficit, while the auditory impairment is due to loss of non-neuronal KCC3 expression. Furthermore, we demonstrate that KCC3 plays an essential role in inflammatory pain pathways. Finally, we observed hypoplasia of the corpus callosum in both mouse mutants and a marked decrease in axonal tracts serving the auditory cortex in only the general deletion mutant. Together, these results establish KCC3 as an important player in both central and peripheral nervous system maintenance.
Figures
Similar articles
-
Temporal manipulation of KCC3 expression in juvenile or adult mice suggests irreversible developmental deficit in hereditary motor sensory neuropathy with agenesis of the corpus callosum.Am J Physiol Cell Physiol. 2021 May 1;320(5):C722-C730. doi: 10.1152/ajpcell.00594.2020. Epub 2021 Feb 17. Am J Physiol Cell Physiol. 2021. PMID: 33596149 Free PMC article.
-
Deletion of KCC3 in parvalbumin neurons leads to locomotor deficit in a conditional mouse model of peripheral neuropathy associated with agenesis of the corpus callosum.Behav Brain Res. 2014 Nov 1;274:128-36. doi: 10.1016/j.bbr.2014.08.005. Epub 2014 Aug 10. Behav Brain Res. 2014. PMID: 25116249 Free PMC article.
-
Transit defect of potassium-chloride Co-transporter 3 is a major pathogenic mechanism in hereditary motor and sensory neuropathy with agenesis of the corpus callosum.J Biol Chem. 2011 Aug 12;286(32):28456-65. doi: 10.1074/jbc.M111.226894. Epub 2011 May 31. J Biol Chem. 2011. PMID: 21628467 Free PMC article.
-
A role for KCC3 in maintaining cell volume of peripheral nerve fibers.Neurochem Int. 2019 Feb;123:114-124. doi: 10.1016/j.neuint.2018.01.009. Epub 2018 Jan 31. Neurochem Int. 2019. PMID: 29366908 Free PMC article. Review.
-
Hereditary motor and sensory neuropathy with agenesis of the corpus callosum.Ann Neurol. 2003 Jul;54(1):9-18. doi: 10.1002/ana.77777. Ann Neurol. 2003. PMID: 12838516 Review.
Cited by
-
Physiology of SLC12 transporters: lessons from inherited human genetic mutations and genetically engineered mouse knockouts.Am J Physiol Cell Physiol. 2013 Apr 15;304(8):C693-714. doi: 10.1152/ajpcell.00350.2012. Epub 2013 Jan 16. Am J Physiol Cell Physiol. 2013. PMID: 23325410 Free PMC article. Review.
-
The WNK-SPAK/OSR1 Kinases and the Cation-Chloride Cotransporters as Therapeutic Targets for Neurological Diseases.Aging Dis. 2019 Jun 1;10(3):626-636. doi: 10.14336/AD.2018.0928. eCollection 2019 Jun. Aging Dis. 2019. PMID: 31165006 Free PMC article. Review.
-
The role of SLC12A family of cation-chloride cotransporters and drug discovery methodologies.J Pharm Anal. 2023 Dec;13(12):1471-1495. doi: 10.1016/j.jpha.2023.09.002. Epub 2023 Sep 9. J Pharm Anal. 2023. PMID: 38223443 Free PMC article. Review.
-
Temporal manipulation of KCC3 expression in juvenile or adult mice suggests irreversible developmental deficit in hereditary motor sensory neuropathy with agenesis of the corpus callosum.Am J Physiol Cell Physiol. 2021 May 1;320(5):C722-C730. doi: 10.1152/ajpcell.00594.2020. Epub 2021 Feb 17. Am J Physiol Cell Physiol. 2021. PMID: 33596149 Free PMC article.
-
Peripheral motor neuropathy is associated with defective kinase regulation of the KCC3 cotransporter.Sci Signal. 2016 Aug 2;9(439):ra77. doi: 10.1126/scisignal.aae0546. Sci Signal. 2016. PMID: 27485015 Free PMC article.
References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. - PubMed
-
- Bishop KM, Wahlsten D. Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res. 1999;815:358–366. - PubMed
-
- Boettger T, Hübner CA, Maier H, Rust MB, Beck FX, Jentsch TJ. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. - PubMed
-
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Völkl H, Hübner CA, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. EMBO J. 2003;22:5422–5434. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
