Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction
- PMID: 22286174
- PMCID: PMC3288805
- DOI: 10.1038/nn.3040
Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction
Abstract
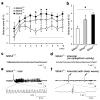
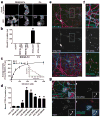
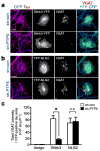
Balanced development of excitatory and inhibitory synapses is required for normal brain function, and an imbalance in this development may underlie the pathogenesis of many neuropsychiatric disorders. Compared with the many identified trans-synaptic adhesion complexes that organize excitatory synapses, little is known about the organizers that are specific for inhibitory synapses. We found that Slit and NTRK-like family member 3 (Slitrk3) actS as a postsynaptic adhesion molecule that selectively regulates inhibitory synapse development via trans-interaction with axonal tyrosine phosphatase receptor PTPδ. When expressed in fibroblasts, Slitrk3 triggered only inhibitory presynaptic differentiation in contacting axons of co-cultured rat hippocampal neurons. Recombinant Slitrk3 preferentially localized to inhibitory postsynaptic sites. Slitrk3-deficient mice exhibited decreases in inhibitory, but not excitatory, synapse number and function in hippocampal CA1 neurons and exhibited increased seizure susceptibility and spontaneous epileptiform activity. Slitrk3 required trans-interaction with axonal PTPδ to induce inhibitory presynaptic differentiation. These results identify Slitrk3-PTPδ as an inhibitory-specific trans-synaptic organizing complex that is required for normal functional GABAergic synapse development.
Figures

Similar articles
-
Trans-synaptic adhesion between NGL-3 and LAR regulates the formation of excitatory synapses.Nat Neurosci. 2009 Apr;12(4):428-37. doi: 10.1038/nn.2279. Epub 2009 Mar 1. Nat Neurosci. 2009. PMID: 19252495
-
Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule.J Neurosci. 2012 Feb 22;32(8):2588-600. doi: 10.1523/JNEUROSCI.4637-11.2012. J Neurosci. 2012. PMID: 22357843 Free PMC article.
-
Trans-synaptic adhesions between netrin-G ligand-3 (NGL-3) and receptor tyrosine phosphatases LAR, protein-tyrosine phosphatase delta (PTPdelta), and PTPsigma via specific domains regulate excitatory synapse formation.J Biol Chem. 2010 Apr 30;285(18):13966-78. doi: 10.1074/jbc.M109.061127. Epub 2010 Feb 6. J Biol Chem. 2010. PMID: 20139422 Free PMC article.
-
LAR-RPTPs: synaptic adhesion molecules that shape synapse development.Trends Cell Biol. 2013 Oct;23(10):465-75. doi: 10.1016/j.tcb.2013.07.004. Epub 2013 Aug 3. Trends Cell Biol. 2013. PMID: 23916315 Review.
-
GABA and neuroligin signaling: linking synaptic activity and adhesion in inhibitory synapse development.Curr Opin Neurobiol. 2008 Feb;18(1):77-83. doi: 10.1016/j.conb.2008.05.008. Epub 2008 May 29. Curr Opin Neurobiol. 2008. PMID: 18513949 Free PMC article. Review.
Cited by
-
The adhesion protein IgSF9b is coupled to neuroligin 2 via S-SCAM to promote inhibitory synapse development.J Cell Biol. 2013 Jun 10;201(6):929-44. doi: 10.1083/jcb.201209132. J Cell Biol. 2013. PMID: 23751499 Free PMC article.
-
SLITRK3 expression correlation to gastrointestinal stromal tumor risk rating and prognosis.World J Gastroenterol. 2015 Jul 21;21(27):8398-407. doi: 10.3748/wjg.v21.i27.8398. World J Gastroenterol. 2015. PMID: 26217092 Free PMC article.
-
Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases.Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4057-62. doi: 10.1073/pnas.1209881110. Epub 2013 Jan 23. Proc Natl Acad Sci U S A. 2013. PMID: 23345436 Free PMC article.
-
Structure of Slitrk2-PTPδ complex reveals mechanisms for splicing-dependent trans-synaptic adhesion.Sci Rep. 2015 May 19;5:9686. doi: 10.1038/srep09686. Sci Rep. 2015. PMID: 25989451 Free PMC article.
-
SLITRK1-mediated noradrenergic projection suppression in the neonatal prefrontal cortex.Commun Biol. 2022 Sep 9;5(1):935. doi: 10.1038/s42003-022-03891-y. Commun Biol. 2022. PMID: 36085162 Free PMC article.
References
-
- Huang ZJ, Di Cristo G, Ango F. Development of GABA innervation in the cerebral and cerebellar cortices. Nat Rev Neurosci. 2007;8:673–86. - PubMed
-
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–9. - PubMed
-
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. - PubMed
-
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–9. - PubMed
-
- Cline H. Synaptogenesis: a balancing act between excitation and inhibition. Curr Biol. 2005;15:R203–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

