The long quest for neonatal screening for severe combined immunodeficiency
- PMID: 22277203
- PMCID: PMC3294102
- DOI: 10.1016/j.jaci.2011.12.964
The long quest for neonatal screening for severe combined immunodeficiency
Abstract
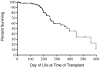
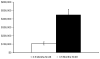
Early recognition of severe combined immunodeficiency (SCID) is a pediatric emergency because a diagnosis before live vaccines or nonirradiated blood products are given and before development of infections permits lifesaving unfractionated HLA-identical or T cell-depleted haploidentical hematopoietic stem cell transplantation, enzyme replacement therapy, or gene therapy. The need for newborn screening for this condition has been recognized for the past 15 years. However, implementation of screening required development of an assay for T-cell lymphopenia that could be performed on dried bloodspots routinely collected from newborn infants for the past 48 years. This was accomplished 6 years ago, and there have already been 7 successful pilot studies. A recommendation to add SCID to the routine newborn-screening panel was approved by the Secretary's Advisory Committee on Heritable Disorders of Newborns and Children in 2010 and was soon after approved by the Secretary of Health and Human Services. It is important for allergists, immunologists, and other health care providers to take an active role in promoting newborn screening for SCID and other T-lymphocyte abnormalities in their states. Even more important will be their roles in establishing accurate diagnoses for infants with positive screen results and in ensuring that they are given the best possible treatment.
Copyright © 2012 American Academy of Allergy, Asthma & Immunology. Published by Mosby, Inc. All rights reserved.
Figures

Similar articles
-
History and current status of newborn screening for severe combined immunodeficiency.Semin Perinatol. 2015 Apr;39(3):194-205. doi: 10.1053/j.semperi.2015.03.004. Epub 2015 Apr 30. Semin Perinatol. 2015. PMID: 25937517 Free PMC article. Review.
-
The case for severe combined immunodeficiency (SCID) and T cell lymphopenia newborn screening: saving lives…one at a time.Immunol Res. 2020 Feb;68(1):48-53. doi: 10.1007/s12026-020-09117-9. Immunol Res. 2020. PMID: 32128663
-
The Wisconsin approach to newborn screening for severe combined immunodeficiency.J Allergy Clin Immunol. 2012 Mar;129(3):622-7. doi: 10.1016/j.jaci.2011.12.004. Epub 2012 Jan 11. J Allergy Clin Immunol. 2012. PMID: 22244594
-
Newborn screening for severe combined immunodeficiency in 11 screening programs in the United States.JAMA. 2014 Aug 20;312(7):729-38. doi: 10.1001/jama.2014.9132. JAMA. 2014. PMID: 25138334 Free PMC article.
-
The case for mandatory newborn screening for severe combined immunodeficiency (SCID).J Clin Immunol. 2014 May;34(4):393-7. doi: 10.1007/s10875-014-0029-0. Epub 2014 Apr 2. J Clin Immunol. 2014. PMID: 24691999 Review.
Cited by
-
Paving the way in implementation of SCID newborn screening in developing nations: feasibility study and strategies to move forward in Malaysia.Front Immunol. 2024 Jun 25;15:1400247. doi: 10.3389/fimmu.2024.1400247. eCollection 2024. Front Immunol. 2024. PMID: 38983864 Free PMC article.
-
DNA recombination defects in Kuwait: Clinical, immunologic and genetic profile.Clin Immunol. 2018 Feb;187:68-75. doi: 10.1016/j.clim.2017.10.006. Epub 2017 Oct 16. Clin Immunol. 2018. PMID: 29051008 Free PMC article.
-
History and current status of newborn screening for severe combined immunodeficiency.Semin Perinatol. 2015 Apr;39(3):194-205. doi: 10.1053/j.semperi.2015.03.004. Epub 2015 Apr 30. Semin Perinatol. 2015. PMID: 25937517 Free PMC article. Review.
-
A novel deletion mutation in IL2RG gene results in X-linked severe combined immunodeficiency with an atypical phenotype.Immunogenetics. 2017 Jan;69(1):29-38. doi: 10.1007/s00251-016-0949-3. Epub 2016 Aug 26. Immunogenetics. 2017. PMID: 27566612
-
B-cell reconstitution for SCID: should a conditioning regimen be used in SCID treatment?J Allergy Clin Immunol. 2013 Apr;131(4):994-1000. doi: 10.1016/j.jaci.2013.01.047. Epub 2013 Mar 5. J Allergy Clin Immunol. 2013. PMID: 23465660 Free PMC article. Review.
References
-
- Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57. - PubMed
-
- Russell SM, Tayebi N, Nakajima H, Riedy MC, Roberts JL, Aman MJ, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Sci. 1995;270:797–800. - PubMed
-
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20(4):394–7. - PubMed
-
- Schwarz K, Gauss GH, Ludwig L, Pannicke U, Li Z, Lindner D, et al. RAG mutations in human B cell-negative SCID. Sci. 1996;274:97–9. - PubMed
-
- Moshous D, Callebaut I, de Chasseval R, Corneo B, Cavazzana-Calvo M, Le Deist F, et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105(2):177–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

