Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids
- PMID: 22197934
- PMCID: PMC3267847
- DOI: 10.1038/ng.1027
Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids
Abstract
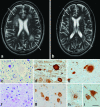
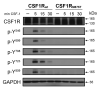
Hereditary diffuse leukoencephalopathy with spheroids (HDLS) is an autosomal-dominant central nervous system white-matter disease with variable clinical presentations, including personality and behavioral changes, dementia, depression, parkinsonism, seizures and other phenotypes. We combined genome-wide linkage analysis with exome sequencing and identified 14 different mutations affecting the tyrosine kinase domain of the colony stimulating factor 1 receptor (encoded by CSF1R) in 14 families with HDLS. In one kindred, we confirmed the de novo occurrence of the mutation. Follow-up sequencing identified an additional CSF1R mutation in an individual diagnosed with corticobasal syndrome. In vitro, CSF-1 stimulation resulted in rapid autophosphorylation of selected tyrosine residues in the kinase domain of wild-type but not mutant CSF1R, suggesting that HDLS may result from partial loss of CSF1R function. As CSF1R is a crucial mediator of microglial proliferation and differentiation in the brain, our findings suggest an important role for microglial dysfunction in HDLS pathogenesis.
Figures


Similar articles
-
CSF1R mutations link POLD and HDLS as a single disease entity.Neurology. 2013 Mar 12;80(11):1033-40. doi: 10.1212/WNL.0b013e31828726a7. Epub 2013 Feb 13. Neurology. 2013. PMID: 23408870 Free PMC article.
-
Genetic analysis of inherited leukodystrophies: genotype-phenotype correlations in the CSF1R gene.JAMA Neurol. 2013 Jul;70(7):875-882. doi: 10.1001/jamaneurol.2013.698. JAMA Neurol. 2013. PMID: 23649896 Free PMC article.
-
A family with hereditary diffuse leukoencephalopathy with spheroids caused by a novel c.2442+2T>C mutation in the CSF1R gene.J Neurol Sci. 2016 Aug 15;367:349-55. doi: 10.1016/j.jns.2016.06.013. Epub 2016 Jun 7. J Neurol Sci. 2016. PMID: 27423618
-
Hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS): update on molecular genetics.Neurol Sci. 2016 Sep;37(9):1565-9. doi: 10.1007/s10072-016-2634-6. Epub 2016 Jun 23. Neurol Sci. 2016. PMID: 27338940 Review.
-
[Neuropathology of hereditary diffuse leukoencephalopathy with axonal spheroids (HDLS)].Rinsho Shinkeigaku. 2014;54(12):1165-7. doi: 10.5692/clinicalneurol.54.1165. Rinsho Shinkeigaku. 2014. PMID: 25672734 Review. Japanese.
Cited by
-
IL-34 and CSF-1: similarities and differences.J Bone Miner Metab. 2013 Sep;31(5):486-95. doi: 10.1007/s00774-013-0476-3. Epub 2013 Jun 6. J Bone Miner Metab. 2013. PMID: 23740288 Review.
-
Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo.Sci Adv. 2020 Jun 26;6(26):eaba3239. doi: 10.1126/sciadv.aba3239. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32637606 Free PMC article.
-
Variants Affecting the C-Terminal of CSF1R Cause Congenital Vertebral Malformation Through a Gain-of-Function Mechanism.Front Cell Dev Biol. 2021 Mar 19;9:641133. doi: 10.3389/fcell.2021.641133. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33816491 Free PMC article.
-
Fractalkine-Dependent Microglial Pruning of Viable Oligodendrocyte Progenitor Cells Regulates Myelination.Cell Rep. 2020 Aug 18;32(7):108047. doi: 10.1016/j.celrep.2020.108047. Cell Rep. 2020. PMID: 32814050 Free PMC article.
-
Csf1 Deficiency Dysregulates Glial Responses to Demyelination and Disturbs CNS White Matter Remyelination.Cells. 2019 Dec 31;9(1):99. doi: 10.3390/cells9010099. Cells. 2019. PMID: 31906095 Free PMC article.
References
-
- Axelsson R, Roytta M, Sourander P, Akesson HO, Andersen O. Hereditary diffuse leucoencephalopathy with spheroids. Acta Psychiatr Scand Suppl. 1984;314:1–65. - PubMed
-
- Baba Y, et al. Hereditary diffuse leukoencephalopathy with spheroids: clinical, pathologic and genetic studies of a new kindred. Acta Neuropathol. 2006;111:300–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 AG 21886-01S1/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 NS072187/NS/NINDS NIH HHS/United States
- RC2 NS070276/NS/NINDS NIH HHS/United States
- R01 NS065782/NS/NINDS NIH HHS/United States
- U24 AG021886-11/AG/NIA NIH HHS/United States
- R01 AG026251/AG/NIA NIH HHS/United States
- 1RC2NS070276/NS/NINDS NIH HHS/United States
- P30 AG 10133/AG/NIA NIH HHS/United States
- R01 NS057567/NS/NINDS NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

