Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72
- PMID: 22083254
- PMCID: PMC3277860
- DOI: 10.1007/s00401-011-0907-y
Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72
Abstract
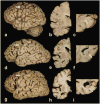
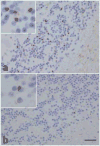
Frontotemporal dementia (FTD) and amyotrophic lateral sclerosis (ALS) are part of a disease spectrum associated with TDP-43 pathology. Strong evidence supporting this is the existence of kindreds with family members affected by FTD, ALS or mixed features of FTD and ALS, referred to as FTD-MND. Some of these families have linkage to chromosome 9, with hexanucleotide expansion mutation in a noncoding region of C9ORF72. Discovery of the mutation defines c9FTD/ALS. Prior to discovery of mutations in C9ORF72, it was assumed that TDP-43 pathology in c9FTD/ALS was uniform. In this study, we examined the neuropathology and clinical features of 20 cases of c9FTD/ALS from a brain bank for neurodegenerative disorders. Included are six patients clinically diagnosed with ALS, eight FTD, one FTD-MND and four Alzheimer-type dementia. Clinical information was unavailable for one patient. Pathologically, the cases all had TDP-43 pathology, but there were three major pathologic groups: ALS, FTLD-MND and FTLD-TDP. The ALS cases were morphologically similar to typical sporadic ALS with almost no extramotor TDP-43 pathology; all had oligodendroglial cytoplasmic inclusions. The FTLD-MND showed predominantly Mackenzie Type 3 TDP-43 pathology, and all had ALS-like pathology in motor neurons, but more extensive extramotor pathology, with oligodendroglial cytoplasmic inclusions and infrequent hippocampal sclerosis. The FTLD-TDP cases had several features similar to FTLD-TDP due to mutations in the gene for progranulin, including Mackenzie Type 1 TDP-43 pathology with neuronal intranuclear inclusions and hippocampal sclerosis. FTLD-TDP patients were older and some were thought to have Alzheimer-type dementia. In addition to the FTD and ALS clinical presentations, the present study shows that c9FTD/ALS can have other presentations, possibly related to age of onset and the presence of hippocampal sclerosis. Moreover, there is pathologic heterogeneity not only between ALS and FTLD, but also within the FTLD group. Further studies are needed to address the molecular mechanism of clinical and pathological heterogeneity of c9FTD/ALS due to mutations in C9ORF72.
Figures


Comment in
-
C9ORF72, the new gene on the block, causes C9FTD/ALS: new insights provided by neuropathology.Acta Neuropathol. 2011 Dec;122(6):653-5. doi: 10.1007/s00401-011-0919-7. Acta Neuropathol. 2011. PMID: 22101324 Free PMC article. No abstract available.
-
The C9ORF72 syndrome: implications for clinical practice.J Neurol. 2012 Apr;259(4):794-6. doi: 10.1007/s00415-012-6479-5. J Neurol. 2012. PMID: 22430253 No abstract available.
Similar articles
-
p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS.Acta Neuropathol. 2011 Dec;122(6):691-702. doi: 10.1007/s00401-011-0911-2. Epub 2011 Nov 19. Acta Neuropathol. 2011. PMID: 22101323
-
Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p.Brain. 2012 Mar;135(Pt 3):709-22. doi: 10.1093/brain/awr354. Epub 2012 Feb 17. Brain. 2012. PMID: 22344582 Free PMC article.
-
Molecular Mechanisms of Neurodegeneration Related to C9orf72 Hexanucleotide Repeat Expansion.Behav Neurol. 2019 Jan 15;2019:2909168. doi: 10.1155/2019/2909168. eCollection 2019. Behav Neurol. 2019. PMID: 30774737 Free PMC article. Review.
-
Clinical and neuropathological features of ALS/FTD with TIA1 mutations.Acta Neuropathol Commun. 2017 Dec 7;5(1):96. doi: 10.1186/s40478-017-0493-x. Acta Neuropathol Commun. 2017. PMID: 29216908 Free PMC article.
-
Prevalence of brain and spinal cord inclusions, including dipeptide repeat proteins, in patients with the C9ORF72 hexanucleotide repeat expansion: a systematic neuropathological review.Neuropathol Appl Neurobiol. 2016 Oct;42(6):547-60. doi: 10.1111/nan.12284. Neuropathol Appl Neurobiol. 2016. PMID: 26373655 Review.
Cited by
-
Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72.Brain. 2012 Mar;135(Pt 3):765-83. doi: 10.1093/brain/aws004. Brain. 2012. PMID: 22366793 Free PMC article.
-
C9ORF72 repeat expansion in clinical and neuropathologic frontotemporal dementia cohorts.Neurology. 2012 Sep 4;79(10):995-1001. doi: 10.1212/WNL.0b013e3182684634. Epub 2012 Aug 8. Neurology. 2012. PMID: 22875086 Free PMC article.
-
Distinct TDP-43 pathology in ALS patients with ataxin 2 intermediate-length polyQ expansions.Acta Neuropathol. 2012 Aug;124(2):221-30. doi: 10.1007/s00401-012-0985-5. Epub 2012 Apr 21. Acta Neuropathol. 2012. PMID: 22526021 Free PMC article.
-
Karyopherin abnormalities in neurodegenerative proteinopathies.Brain. 2021 Nov 29;144(10):2915-2932. doi: 10.1093/brain/awab201. Brain. 2021. PMID: 34019093 Free PMC article. Review.
-
Genetic screening of a large series of North American sporadic and familial frontotemporal dementia cases.Alzheimers Dement. 2020 Jan;16(1):118-130. doi: 10.1002/alz.12011. Alzheimers Dement. 2020. PMID: 31914217 Free PMC article.
References
-
- Arai T, Hasegawa M, Akiyama H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01-AG17216/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 NS072187-01/NS/NINDS NIH HHS/United States
- R01-AG37491/AG/NIA NIH HHS/United States
- P50-AG16574/AG/NIA NIH HHS/United States
- R01-AG26251/AG/NIA NIH HHS/United States
- R01 AG026251/AG/NIA NIH HHS/United States
- P01 AG017216/AG/NIA NIH HHS/United States
- P50-NS72187/NS/NINDS NIH HHS/United States
- P50 NS072187/NS/NINDS NIH HHS/United States
- R01 NS065782/NS/NINDS NIH HHS/United States
- R01-NS65782/NS/NINDS NIH HHS/United States
- R01 AG037491/AG/NIA NIH HHS/United States
- P01 AG003949/AG/NIA NIH HHS/United States
- R01-AG15866/AG/NIA NIH HHS/United States
- P01-AG03949/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

