Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis
- PMID: 21483807
- PMCID: PMC3069110
- DOI: 10.1371/journal.pgen.1001352
Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis
Abstract
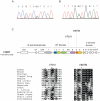
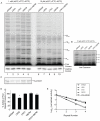
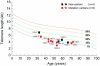
The telomerase reverse transcriptase synthesizes new telomeres onto chromosome ends by copying from a short template within its integral RNA component. During telomere synthesis, telomerase adds multiple short DNA repeats successively, a property known as repeat addition processivity. However, the consequences of defects in processivity on telomere length maintenance are not fully known. Germline mutations in telomerase cause haploinsufficiency in syndromes of telomere shortening, which most commonly manifest in the age-related disease idiopathic pulmonary fibrosis. We identified two pulmonary fibrosis families that share two non-synonymous substitutions in the catalytic domain of the telomerase reverse transcriptase gene hTERT: V791I and V867M. The two variants fell on the same hTERT allele and were associated with telomere shortening. Genealogy suggested that the pedigrees shared a single ancestor from the nineteenth century, and genetic studies confirmed the two families had a common founder. Functional studies indicated that, although the double mutant did not dramatically affect first repeat addition, hTERT V791I-V867M showed severe defects in telomere repeat addition processivity in vitro. Our data identify an ancestral mutation in telomerase with a novel loss-of-function mechanism. They indicate that telomere repeat addition processivity is a critical determinant of telomere length and telomere-mediated disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
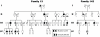
Similar articles
-
Telomerase mutations in families with idiopathic pulmonary fibrosis.N Engl J Med. 2007 Mar 29;356(13):1317-26. doi: 10.1056/NEJMoa066157. N Engl J Med. 2007. PMID: 17392301
-
hTERT mutations associated with idiopathic pulmonary fibrosis affect telomerase activity, telomere length, and cell growth by distinct mechanisms.Aging Cell. 2012 Jun;11(3):482-90. doi: 10.1111/j.1474-9726.2012.00810.x. Epub 2012 Mar 19. Aging Cell. 2012. PMID: 22364217
-
The C terminus of the human telomerase reverse transcriptase is a determinant of enzyme processivity.Nucleic Acids Res. 2003 Jul 15;31(14):4059-70. doi: 10.1093/nar/gkg437. Nucleic Acids Res. 2003. PMID: 12853623 Free PMC article.
-
Short telomeres in pulmonary fibrosis: from genetics to clinical significance.Pneumologia. 2015 Jan-Mar;64(1):8, 11-3. Pneumologia. 2015. PMID: 26016050 Review.
-
Telomerase and idiopathic pulmonary fibrosis.Mutat Res. 2012 Feb 1;730(1-2):52-8. doi: 10.1016/j.mrfmmm.2011.10.013. Epub 2011 Nov 4. Mutat Res. 2012. PMID: 22079513 Free PMC article. Review.
Cited by
-
A persistent variant telomere sequence in a human pedigree.Nat Commun. 2024 Jun 1;15(1):4681. doi: 10.1038/s41467-024-49072-9. Nat Commun. 2024. PMID: 38824190 Free PMC article.
-
Many disease-associated variants of hTERT retain high telomerase enzymatic activity.Nucleic Acids Res. 2013 Oct;41(19):8969-78. doi: 10.1093/nar/gkt653. Epub 2013 Jul 30. Nucleic Acids Res. 2013. PMID: 23901009 Free PMC article.
-
Familial Pulmonary Fibrosis: Genetic Features and Clinical Implications.Chest. 2021 Nov;160(5):1764-1773. doi: 10.1016/j.chest.2021.06.037. Epub 2021 Jun 26. Chest. 2021. PMID: 34186035 Free PMC article. Review.
-
A translocation-defective telomerase with low levels of activity and processivity stabilizes short telomeres and confers immortalization.Mol Biol Cell. 2013 May;24(9):1469-79. doi: 10.1091/mbc.E12-12-0889. Epub 2013 Feb 27. Mol Biol Cell. 2013. PMID: 23447707 Free PMC article.
-
Telomere DNA G-quadruplex folding within actively extending human telomerase.Proc Natl Acad Sci U S A. 2019 May 7;116(19):9350-9359. doi: 10.1073/pnas.1814777116. Epub 2019 Apr 24. Proc Natl Acad Sci U S A. 2019. PMID: 31019071 Free PMC article.
References
-
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. - PubMed
-
- Greider CW, Blackburn EH. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987;51:887–898. - PubMed
-
- Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. - PubMed
-
- Lingner J, Hughes TR, Shevchenko A, Mann M, Lundblad V, et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. - PubMed
-
- Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

