Nitric oxide and redox mechanisms in the immune response
- PMID: 21233414
- PMCID: PMC3100761
- DOI: 10.1189/jlb.1010550
Nitric oxide and redox mechanisms in the immune response
Abstract
The role of redox molecules, such as NO and ROS, as key mediators of immunity has recently garnered renewed interest and appreciation. To regulate immune responses, these species trigger the eradication of pathogens on the one hand and modulate immunosuppression during tissue-restoration and wound-healing processes on the other. In the acidic environment of the phagosome, a variety of RNS and ROS is produced, thereby providing a cauldron of redox chemistry, which is the first line in fighting infection. Interestingly, fluctuations in the levels of these same reactive intermediates orchestrate other phases of the immune response. NO activates specific signal transduction pathways in tumor cells, endothelial cells, and monocytes in a concentration-dependent manner. As ROS can react directly with NO-forming RNS, NO bioavailability and therefore, NO response(s) are changed. The NO/ROS balance is also important during Th1 to Th2 transition. In this review, we discuss the chemistry of NO and ROS in the context of antipathogen activity and immune regulation and also discuss similarities and differences between murine and human production of these intermediates.
Figures

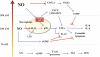
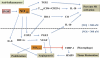
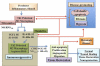

Similar articles
-
Redox signalling: from nitric oxide to oxidized lipids.Biochem Soc Symp. 2004;(71):107-20. doi: 10.1042/bss0710107. Biochem Soc Symp. 2004. PMID: 15777016 Review.
-
Nitric oxide and cell signaling: modulation of redox tone and protein modification.Amino Acids. 2003 Dec;25(3-4):313-21. doi: 10.1007/s00726-003-0019-7. Epub 2003 Aug 28. Amino Acids. 2003. PMID: 14661093 Review.
-
Redox regulation of the immune response.Redox Rep. 2013;18(3):88-94. doi: 10.1179/1351000213Y.0000000044. Epub 2013 Apr 19. Redox Rep. 2013. PMID: 23601165 Free PMC article. Review.
-
Subcellular localization of oxidants and redox modulation of endothelial nitric oxide synthase.Circ J. 2012;76(11):2497-512. doi: 10.1253/circj.cj-12-1207. Epub 2012 Oct 18. Circ J. 2012. PMID: 23075817 Review.
-
Free radicals and antioxidants in normal physiological functions and human disease.Int J Biochem Cell Biol. 2007;39(1):44-84. doi: 10.1016/j.biocel.2006.07.001. Epub 2006 Aug 4. Int J Biochem Cell Biol. 2007. PMID: 16978905 Review.
Cited by
-
Microneedle Delivery of an Adjuvanted Microparticulate Vaccine Induces High Antibody Levels in Mice Vaccinated against Coronavirus.Vaccines (Basel). 2022 Sep 7;10(9):1491. doi: 10.3390/vaccines10091491. Vaccines (Basel). 2022. PMID: 36146568 Free PMC article.
-
P19 a Parthenin Analog Induces Cell Lineage Dependent Apoptotic and Immunomodulatory Signaling in Acute Lymphoid Leukemia Cells.Int J Mol Cell Med. 2023;12(1):1-17. doi: 10.22088/IJMCM.BUMS.12.1.1. Int J Mol Cell Med. 2023. PMID: 37942260 Free PMC article.
-
Methicillin-resistant Staphylococcus aureus bacterial nitric-oxide synthase affects antibiotic sensitivity and skin abscess development.J Biol Chem. 2013 Mar 1;288(9):6417-26. doi: 10.1074/jbc.M112.448738. Epub 2013 Jan 15. J Biol Chem. 2013. PMID: 23322784 Free PMC article.
-
Plasma medicine for neuroscience-an introduction.Chin Neurosurg J. 2019 Oct 19;5:25. doi: 10.1186/s41016-019-0172-9. eCollection 2019. Chin Neurosurg J. 2019. PMID: 32922924 Free PMC article. Review.
-
Arginase as a Potential Biomarker of Disease Progression: A Molecular Imaging Perspective.Int J Mol Sci. 2020 Jul 25;21(15):5291. doi: 10.3390/ijms21155291. Int J Mol Sci. 2020. PMID: 32722521 Free PMC article. Review.
References
-
- Colton C., Gilbert D. (1999) Reactive Oxygen Species in Biological Systems, New York, NY, USA, Springer
-
- Halliwell B., Gutteridge J. M. C. (1999) Free Radicals in Biology and Medicine, Oxford, UK, Oxford University Press
-
- Forman H. J., Fukuto J. M., Torres M. (2004) Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. Am. J. Physiol. Cell Physiol. 287, C246–C256 - PubMed
-
- Forman H. J., Torres M., Fukuto J. (2003) Signal Transduction by Reactive Oxygen and Nitrogen Species: Pathways and Chemical Principles, Dordrecjt, The Netherlands; Boston, MA, USA, Kluwer Academic Publishers
-
- Forman H. J., Torres M., Fukuto J. (2002) Redox signaling. Mol. Cell. Biochem. 234-235, 49–62 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

