The genetics and clinical manifestations of telomere biology disorders
- PMID: 21189492
- PMCID: PMC3825100
- DOI: 10.1097/GIM.0b013e3181f415b5
The genetics and clinical manifestations of telomere biology disorders
Abstract
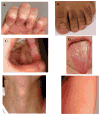
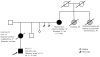
Telomere biology disorders are a complex set of illnesses defined by the presence of very short telomeres. Individuals with classic dyskeratosis congenita have the most severe phenotype, characterized by the triad of nail dystrophy, abnormal skin pigmentation, and oral leukoplakia. More significantly, these individuals are at very high risk of bone marrow failure, cancer, and pulmonary fibrosis. A mutation in one of six different telomere biology genes can be identified in 50–60% of these individuals. DKC1, TERC, TERT, NOP10, and NHP2 encode components of telomerase or a telomerase-associated factor and TINF2, a telomeric protein. Progressively shorter telomeres are inherited from generation to generation in autosomal dominant dyskeratosis congenita, resulting in disease anticipation. Up to 10% of individuals with apparently acquired aplastic anemia or idiopathic pulmonary fibrosis also have short telomeres and mutations in TERC or TERT. Similar findings have been seen in individuals with liver fibrosis or acute myelogenous leukemia. This report reviews basic aspects of telomere biology and telomere length measurement, and the clinical and genetic features of those disorders that constitute our current understanding of the spectrum of illness caused by defects in telomere biology. We also suggest a grouping schema for the telomere disorders.
Conflict of interest statement
Sharon A. Savage, M.D.: No commercial associations to disclose.
Alison A. Bertuch, M.D., Ph.D.: No commercial associations to disclose
Figures
Similar articles
-
Recent progress in dyskeratosis congenita.Int J Hematol. 2010 Oct;92(3):419-24. doi: 10.1007/s12185-010-0695-5. Epub 2010 Oct 1. Int J Hematol. 2010. PMID: 20882440 Review.
-
Dyskeratosis congenita.Hematology Am Soc Hematol Educ Program. 2011;2011:480-6. doi: 10.1182/asheducation-2011.1.480. Hematology Am Soc Hematol Educ Program. 2011. PMID: 22160078 Review.
-
Dyskeratosis congenita, stem cells and telomeres.Biochim Biophys Acta. 2009 Apr;1792(4):371-9. doi: 10.1016/j.bbadis.2009.01.010. Epub 2009 Feb 7. Biochim Biophys Acta. 2009. PMID: 19419704 Free PMC article. Review.
-
Proliferative defects in dyskeratosis congenita skin keratinocytes are corrected by expression of the telomerase reverse transcriptase, TERT, or by activation of endogenous telomerase through expression of papillomavirus E6/E7 or the telomerase RNA component, TERC.Exp Dermatol. 2010 Mar;19(3):279-88. doi: 10.1111/j.1600-0625.2009.00916.x. Epub 2009 Jun 23. Exp Dermatol. 2010. PMID: 19558498 Free PMC article.
-
Short telomeres: from dyskeratosis congenita to sporadic aplastic anemia and malignancy.Transl Res. 2013 Dec;162(6):353-63. doi: 10.1016/j.trsl.2013.05.003. Epub 2013 Jun 1. Transl Res. 2013. PMID: 23732052 Free PMC article. Review.
Cited by
-
Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita.Hum Genet. 2013 Apr;132(4):473-80. doi: 10.1007/s00439-013-1265-8. Epub 2013 Jan 18. Hum Genet. 2013. PMID: 23329068 Free PMC article. Clinical Trial.
-
Telomeres: the beginnings and ends of eukaryotic chromosomes.Exp Cell Res. 2012 Jul 15;318(12):1456-60. doi: 10.1016/j.yexcr.2012.02.015. Epub 2012 Feb 25. Exp Cell Res. 2012. PMID: 22391099 Free PMC article. Review.
-
Transcriptomic Analysis of Conserved Telomere Maintenance Component 1 (CTC1) and Its Association with Leukemia.J Clin Med. 2022 Sep 29;11(19):5780. doi: 10.3390/jcm11195780. J Clin Med. 2022. PMID: 36233645 Free PMC article.
-
Interactions of the human telomere sequence with the nanocavity of the α-hemolysin ion channel reveal structure-dependent electrical signatures for hybrid folds.J Am Chem Soc. 2013 Jun 12;135(23):8562-70. doi: 10.1021/ja400973m. Epub 2013 May 29. J Am Chem Soc. 2013. PMID: 23682802 Free PMC article.
-
Development of metachronous rectal cancers in a young man with dyskeratosis congenita: a case report.J Med Case Rep. 2019 Apr 27;13(1):117. doi: 10.1186/s13256-019-2044-5. J Med Case Rep. 2019. PMID: 31027506 Free PMC article.
References
-
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. - PubMed
-
- Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. - PubMed
-
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579(4):859–862. - PubMed
-
- Robertson JD, Gale RE, Wynn RF, Dougal M, Linch DC, Testa NG, et al. Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. Br J Haematol. 2000;109(2):272–279. - PubMed
-
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

