Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage
- PMID: 20649466
- PMCID: PMC3096496
- DOI: 10.1089/ars.2010.3466
Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage
Abstract
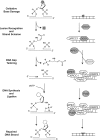
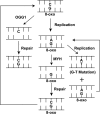
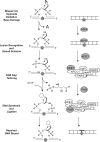
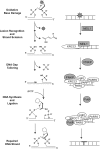
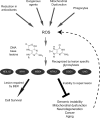
Nuclear and mitochondrial genomes are under continuous assault by a combination of environmentally and endogenously derived reactive oxygen species, inducing the formation and accumulation of mutagenic, toxic, and/or genome-destabilizing DNA lesions. Failure to resolve these lesions through one or more DNA-repair processes is associated with genome instability, mitochondrial dysfunction, neurodegeneration, inflammation, aging, and cancer, emphasizing the importance of characterizing the pathways and proteins involved in the repair of oxidative DNA damage. This review focuses on the repair of oxidative damage-induced lesions in nuclear and mitochondrial DNA mediated by the base excision repair (BER) pathway in mammalian cells. We discuss the multiple BER subpathways that are initiated by one of 11 different DNA glycosylases of three subtypes: (a) bifunctional with an associated β-lyase activity; (b) monofunctional; and (c) bifunctional with an associated β,δ-lyase activity. These three subtypes of DNA glycosylases all initiate BER but yield different chemical intermediates and hence different BER complexes to complete repair. Additionally, we briefly summarize alternate repair events mediated by BER proteins and the role of BER in the repair of mitochondrial DNA damage induced by ROS. Finally, we discuss the relation of BER and oxidative DNA damage in the onset of human disease.
Figures

Similar articles
-
Base excision repair of oxidative DNA damage: from mechanism to disease.Front Biosci (Landmark Ed). 2017 Mar 1;22(9):1493-1522. doi: 10.2741/4555. Front Biosci (Landmark Ed). 2017. PMID: 28199214 Free PMC article. Review.
-
Human DNA glycosylases involved in the repair of oxidatively damaged DNA.Biol Pharm Bull. 2004 Apr;27(4):480-5. doi: 10.1248/bpb.27.480. Biol Pharm Bull. 2004. PMID: 15056851 Review.
-
Transcription coupled base excision repair in mammalian cells: So little is known and so much to uncover.DNA Repair (Amst). 2021 Nov;107:103204. doi: 10.1016/j.dnarep.2021.103204. Epub 2021 Aug 6. DNA Repair (Amst). 2021. PMID: 34390916 Review.
-
Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells.Cell Res. 2008 Jan;18(1):27-47. doi: 10.1038/cr.2008.8. Cell Res. 2008. PMID: 18166975 Free PMC article. Review.
-
Oxidative genome damage and its repair: implications in aging and neurodegenerative diseases.Mech Ageing Dev. 2012 Apr;133(4):157-68. doi: 10.1016/j.mad.2012.01.005. Epub 2012 Jan 31. Mech Ageing Dev. 2012. PMID: 22313689 Free PMC article. Review.
Cited by
-
How Do ROS Induce NETosis? Oxidative DNA Damage, DNA Repair, and Chromatin Decondensation.Biomolecules. 2024 Oct 16;14(10):1307. doi: 10.3390/biom14101307. Biomolecules. 2024. PMID: 39456240 Free PMC article. Review.
-
Function and molecular mechanisms of APE2 in genome and epigenome integrity.Mutat Res Rev Mutat Res. 2021 Jan-Jun;787:108347. doi: 10.1016/j.mrrev.2020.108347. Epub 2020 Nov 16. Mutat Res Rev Mutat Res. 2021. PMID: 34083046 Free PMC article. Review.
-
Base excision repair.Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a012583. doi: 10.1101/cshperspect.a012583. Cold Spring Harb Perspect Biol. 2013. PMID: 23545420 Free PMC article. Review.
-
Complex DNA repair pathways as possible therapeutic targets to overcome temozolomide resistance in glioblastoma.Front Oncol. 2012 Dec 5;2:186. doi: 10.3389/fonc.2012.00186. eCollection 2012. Front Oncol. 2012. PMID: 23227453 Free PMC article.
-
Saccharomyces cerevisiae as a Model System for Eukaryotic Cell Biology, from Cell Cycle Control to DNA Damage Response.Int J Mol Sci. 2022 Oct 1;23(19):11665. doi: 10.3390/ijms231911665. Int J Mol Sci. 2022. PMID: 36232965 Free PMC article. Review.
References
-
- Albright CD. Salganik RI. Craciunescu CN. Mar MH. Zeisel SH. Mitochondrial and microsomal derived reactive oxygen species mediate apoptosis induced by transforming growth factor-beta1 in immortalized rat hepatocytes. J Cell Biochem. 2003;89:254–261. - PubMed
-
- Almeida KH. Sobol RW. Increased specificity and efficiency of base excision repair through complex formation. In: Siede W, editor; Doetsch PW, editor; Kow YW, editor. DNA Damage Recognition. New York: Marcel Dekker; 2005. pp. 33–64.
-
- Bartsch H. Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prevent. 2004;28:385–391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

