Decision checkpoints in the thymus
- PMID: 20644572
- PMCID: PMC3388799
- DOI: 10.1038/ni.1887
Decision checkpoints in the thymus
Erratum in
- Nat Immunol. 2011 Mar;12(3):271
Abstract
The development of T cells in the thymus involves several differentiation and proliferation events, during which hematopoietic precursors give rise to T cells ready to respond to antigen stimulation and undergo effector differentiation. This review addresses signaling and transcriptional checkpoints that control the intrathymic journey of T cell precursors. We focus on the divergence of alphabeta and gammadelta lineage cells and the elaboration of the alphabeta T cell repertoire, with special emphasis on the emergence of transcriptional programs that direct lineage decisions.
Figures
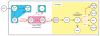


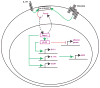
Similar articles
-
Specific Notch receptor-ligand interactions control human TCR-αβ/γδ development by inducing differential Notch signal strength.J Exp Med. 2013 Apr 8;210(4):683-97. doi: 10.1084/jem.20121798. Epub 2013 Mar 25. J Exp Med. 2013. PMID: 23530123 Free PMC article. Clinical Trial.
-
Delta-like 4-mediated Notch signaling is required for early T-cell development in a three-dimensional thymic structure.Eur J Immunol. 2015 Aug;45(8):2252-62. doi: 10.1002/eji.201445123. Epub 2015 Jun 3. Eur J Immunol. 2015. PMID: 25976373
-
A comparative view on vitamin C effects on αβ- versus γδ T-cell activation and differentiation.J Leukoc Biol. 2020 Jun;107(6):1009-1022. doi: 10.1002/JLB.1MR1219-245R. Epub 2020 Feb 7. J Leukoc Biol. 2020. PMID: 32034803 Review.
-
αβ and γδ T cell receptors: Similar but different.J Leukoc Biol. 2020 Jun;107(6):1045-1055. doi: 10.1002/JLB.2MR1219-233R. Epub 2020 Jan 29. J Leukoc Biol. 2020. PMID: 31994778 Review.
-
Lineage divergence at the first TCR-dependent checkpoint: preferential γδ and impaired αβ T cell development in nonobese diabetic mice.J Immunol. 2011 Jan 15;186(2):826-37. doi: 10.4049/jimmunol.1002630. Epub 2010 Dec 10. J Immunol. 2011. PMID: 21148803 Free PMC article.
Cited by
-
Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling.Nat Immunol. 2016 May;17(5):565-73. doi: 10.1038/ni.3419. Epub 2016 Apr 4. Nat Immunol. 2016. PMID: 27043411 Free PMC article.
-
Synergistic effects of interleukin-7 and pre-T cell receptor signaling in human T cell development.J Biol Chem. 2012 Sep 28;287(40):33826-35. doi: 10.1074/jbc.M112.380113. Epub 2012 Aug 2. J Biol Chem. 2012. PMID: 22859301 Free PMC article.
-
TET Proteins in the Spotlight: Emerging Concepts of Epigenetic Regulation in T Cell Biology.Immunohorizons. 2023 Jan 1;7(1):106-115. doi: 10.4049/immunohorizons.2200067. Immunohorizons. 2023. PMID: 36645853 Free PMC article.
-
Neutrophil and T Cell Functions in Patients with Congenital Heart Diseases: A Review.Pediatr Cardiol. 2021 Oct;42(7):1478-1482. doi: 10.1007/s00246-021-02681-3. Epub 2021 Jul 20. Pediatr Cardiol. 2021. PMID: 34282478 Free PMC article. Review.
-
IL-7-Induced Proliferation of Human Naive CD4 T-Cells Relies on Continued Thymic Activity.Front Immunol. 2017 Jan 19;8:20. doi: 10.3389/fimmu.2017.00020. eCollection 2017. Front Immunol. 2017. PMID: 28154568 Free PMC article.
References
-
- Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. - PubMed
-
- Bhandoola A, von Boehmer H, Petrie HT, Zuniga-Pflucker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–689. - PubMed
-
- Feyerabend TB, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

