Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation
- PMID: 20434207
- PMCID: PMC2881594
- DOI: 10.1016/j.cell.2010.04.008
Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation
Erratum in
-
Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation.Cell. 2021 Apr 29;184(9):2520. doi: 10.1016/j.cell.2021.04.018. Cell. 2021. PMID: 33930296 Free PMC article. No abstract available.
Abstract
Extracellular signals regulate protein translation in many cell functions. A key advantage of control at the translational level is the opportunity to regulate protein synthesis within specific cellular subregions. However, little is known about mechanisms that may link extracellular cues to translation with spatial precision. Here, we show that a transmembrane receptor, DCC, forms a binding complex containing multiple translation components, including eukaryotic initiation factors, ribosomal large and small subunits, and monosomes. In neuronal axons and dendrites DCC colocalizes in particles with translation machinery, and newly synthesized protein. The extracellular ligand netrin promoted DCC-mediated translation and disassociation of translation components. The functional and physical association of a cell surface receptor with the translation machinery leads to a generalizable model for localization and extracellular regulation of protein synthesis, based on a transmembrane translation regulation complex.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures


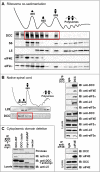
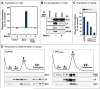
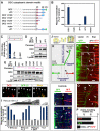
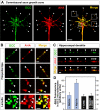

Comment in
-
Anchoring local translation in neurons.Cell. 2010 May 14;141(4):566-8. doi: 10.1016/j.cell.2010.04.031. Cell. 2010. PMID: 20478248 Free PMC article.
Similar articles
-
p120RasGAP Protein Mediates Netrin-1 Protein-induced Cortical Axon Outgrowth and Guidance.J Biol Chem. 2016 Feb 26;291(9):4589-602. doi: 10.1074/jbc.M115.674846. Epub 2015 Dec 28. J Biol Chem. 2016. PMID: 26710849 Free PMC article.
-
The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue.Neuron. 2014 Aug 20;83(4):839-849. doi: 10.1016/j.neuron.2014.07.010. Epub 2014 Aug 7. Neuron. 2014. PMID: 25123307 Free PMC article.
-
Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction.J Neurosci. 2006 Mar 22;26(12):3192-205. doi: 10.1523/JNEUROSCI.3469-05.2006. J Neurosci. 2006. PMID: 16554470 Free PMC article.
-
Signaling mechanism of the netrin-1 receptor DCC in axon guidance.Prog Biophys Mol Biol. 2015 Sep;118(3):153-60. doi: 10.1016/j.pbiomolbio.2015.04.001. Epub 2015 Apr 14. Prog Biophys Mol Biol. 2015. PMID: 25881791 Free PMC article. Review.
-
Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning.Cell Cycle. 2005 Mar;4(3):e131-5. Epub 2005 Mar 18. Cell Cycle. 2005. PMID: 15725728 Review.
Cited by
-
Two-strain, cell-selective protein labeling in mixed bacterial cultures.J Am Chem Soc. 2012 May 23;134(20):8551-6. doi: 10.1021/ja3004667. Epub 2012 May 10. J Am Chem Soc. 2012. PMID: 22575034 Free PMC article.
-
Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target.Wiley Interdiscip Rev Dev Biol. 2015 Jul-Aug;4(4):377-89. doi: 10.1002/wdev.185. Epub 2015 Mar 16. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 25779002 Free PMC article. Review.
-
Spontaneous activity regulates Robo1 transcription to mediate a switch in thalamocortical axon growth.Nat Neurosci. 2012 Jul 8;15(8):1134-43. doi: 10.1038/nn.3160. Nat Neurosci. 2012. PMID: 22772332
-
Distinct recruitment of human eIF4E isoforms to processing bodies and stress granules.BMC Mol Biol. 2016 Aug 30;17(1):21. doi: 10.1186/s12867-016-0072-x. BMC Mol Biol. 2016. PMID: 27578149 Free PMC article.
-
Src inhibits midline axon crossing independent of Frazzled/Deleted in Colorectal Carcinoma (DCC) receptor tyrosine phosphorylation.J Neurosci. 2013 Jan 2;33(1):305-14. doi: 10.1523/JNEUROSCI.2756-12.2013. J Neurosci. 2013. PMID: 23283343 Free PMC article.
References
-
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. - PubMed
-
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. - PubMed
-
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

