A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition
- PMID: 20395263
- PMCID: PMC2859154
- DOI: 10.1093/brain/awq071
A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition
Abstract
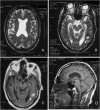
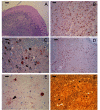
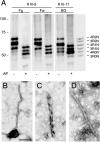
We have studied a family with severe mental retardation characterized by the virtual absence of speech, autism spectrum disorder, epilepsy, late-onset ataxia, weakness and dystonia. Post-mortem examination of two males revealed widespread neuronal loss, with the most striking finding being neuronal and glial tau deposition in a pattern reminiscent of corticobasal degeneration. Electron microscopic examination of isolated tau filaments demonstrated paired helical filaments and ribbon-like structures. Biochemical studies of tau demonstrated a preponderance of 4R tau isoforms. The phenotype was linked to Xq26.3, and further analysis identified an in-frame 9 base pair deletion in the solute carrier family 9, isoform A6 (SLC9A6 gene), which encodes sodium/hydrogen exchanger-6 localized to endosomal vesicles. Sodium/hydrogen exchanger-6 is thought to participate in the targeting of intracellular vesicles and may be involved in recycling synaptic vesicles. The striking tau deposition in our subjects reveals a probable interaction between sodium/proton exchangers and cytoskeletal elements involved in vesicular transport, and raises the possibility that abnormalities of vesicular targeting may play an important role in more common disorders such as Alzheimer's disease and autism spectrum disorders.
Figures
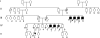
Similar articles
-
X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction.Brain. 2011 Nov;134(Pt 11):3369-83. doi: 10.1093/brain/awr250. Epub 2011 Sep 29. Brain. 2011. PMID: 21964919 Free PMC article.
-
A new family with an SLC9A6 mutation expanding the phenotypic spectrum of Christianson syndrome.Am J Med Genet A. 2016 Aug;170(8):2103-10. doi: 10.1002/ajmg.a.37765. Epub 2016 Jun 3. Am J Med Genet A. 2016. PMID: 27256868
-
A Christianson syndrome-linked deletion mutation (∆(287)ES(288)) in SLC9A6 disrupts recycling endosomal function and elicits neurodegeneration and cell death.Mol Neurodegener. 2016 Sep 2;11(1):63. doi: 10.1186/s13024-016-0129-9. Mol Neurodegener. 2016. PMID: 27590723 Free PMC article.
-
The expanding phenotypic spectrum of female SLC9A6 mutation carriers: a case series and review of the literature.Hum Genet. 2016 Aug;135(8):841-50. doi: 10.1007/s00439-016-1675-5. Epub 2016 May 3. Hum Genet. 2016. PMID: 27142213 Review.
-
Tau mutations in frontotemporal dementia FTDP-17 and their relevance for Alzheimer's disease.Biochim Biophys Acta. 2000 Jul 26;1502(1):110-21. doi: 10.1016/s0925-4439(00)00037-5. Biochim Biophys Acta. 2000. PMID: 10899436 Review.
Cited by
-
Functional Assessment In Vivo of the Mouse Homolog of the Human Ala-9-Ser NHE6 Variant.eNeuro. 2019 Dec 4;6(6):ENEURO.0046-19.2019. doi: 10.1523/ENEURO.0046-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31676550 Free PMC article.
-
Early lysosome defects precede neurodegeneration with amyloid-β and tau aggregation in NHE6-null rat brain.Brain. 2022 Sep 14;145(9):3187-3202. doi: 10.1093/brain/awab467. Brain. 2022. PMID: 34928329 Free PMC article.
-
Traditional and emerging roles for the SLC9 Na+/H+ exchangers.Pflugers Arch. 2014 Jan;466(1):61-76. doi: 10.1007/s00424-013-1408-8. Epub 2013 Dec 12. Pflugers Arch. 2014. PMID: 24337822 Review.
-
X-linked disorders with cerebellar dysgenesis.Orphanet J Rare Dis. 2011 May 15;6:24. doi: 10.1186/1750-1172-6-24. Orphanet J Rare Dis. 2011. PMID: 21569638 Free PMC article. Review.
-
X-linked Angelman-like syndrome caused by Slc9a6 knockout in mice exhibits evidence of endosomal-lysosomal dysfunction.Brain. 2011 Nov;134(Pt 11):3369-83. doi: 10.1093/brain/awr250. Epub 2011 Sep 29. Brain. 2011. PMID: 21964919 Free PMC article.
References
-
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. - PubMed
-
- Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, et al. Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 2001;101:167–73. - PubMed
-
- Armstrong DD. Neuropathology of Rett syndrome. J Child Neurol. 2005;20:747–53. - PubMed
-
- Armstrong RA, Cairns NJ, Lantos PL. A quantitative study of the pathological lesions in the neocortex and hippocampus of twelve patients with corticobasal degeneration. Exp Neurol. 2000;163:348–56. - PubMed
-
- Belfor N, Amici S, Boxer AL, Kramer JH, Gorno-Tempini ML, Rosen HJ, et al. Clinical and neuropsychological features of corticobasal degeneration. Mech Ageing Dev. 2006;127:203–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

