Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly
- PMID: 20159109
- PMCID: PMC2820185
- DOI: 10.1016/j.ajhg.2010.01.011
Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly
Abstract
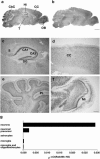
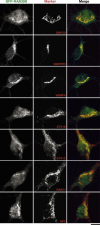
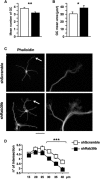
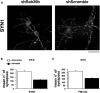
Human Mental Retardation (MR) is a common and highly heterogeneous pediatric disorder affecting around 3% of the general population; at least 215 X-linked MR (XLMR) conditions have been described, and mutations have been identified in 83 different genes, encoding proteins with a variety of function, such as chromatin remodeling, synaptic function, and intracellular trafficking. The small GTPases of the RAB family, which play an essential role in intracellular vesicular trafficking, have been shown to be involved in MR. We report here the identification of mutations in the small GTPase RAB39B gene in two male patients. One mutation in family X (D-23) introduced a stop codon seven amino acids after the start codon (c.21C > A; p.Y7X). A second mutation, in the MRX72 family, altered the 5' splice site (c.215+1G > A) and normal splicing. Neither instance produced a protein. Mutations segregate with the disease in the families, and in some family members intellectual disabilities were associated with autism spectrum disorder, epileptic seizures, and macrocephaly. We show that RAB39B, a novel RAB GTPase of unknown function, is a neuronal-specific protein that is localized to the Golgi compartment. Its downregulation leads to an alteration in the number and morphology of neurite growth cones and a significant reduction in presynaptic buttons, suggesting that RAB39B is required for synapse formation and maintenance. Our results demonstrate developmental and functional neuronal alteration as a consequence of downregulation of RAB39B and emphasize the critical role of vesicular trafficking in the development of neurons and human intellectual abilities.
Copyright (c) 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition.Nat Commun. 2015 Mar 18;6:6504. doi: 10.1038/ncomms7504. Nat Commun. 2015. PMID: 25784538 Free PMC article.
-
Cerebral organoid and mouse models reveal a RAB39b-PI3K-mTOR pathway-dependent dysregulation of cortical development leading to macrocephaly/autism phenotypes.Genes Dev. 2020 Apr 1;34(7-8):580-597. doi: 10.1101/gad.332494.119. Epub 2020 Feb 27. Genes Dev. 2020. PMID: 32115408 Free PMC article.
-
Disruption of PHF21A causes syndromic intellectual disability with craniofacial anomalies, epilepsy, hypotonia, and neurobehavioral problems including autism.Mol Autism. 2019 Oct 22;10:35. doi: 10.1186/s13229-019-0286-0. eCollection 2019. Mol Autism. 2019. PMID: 31649809 Free PMC article.
-
RAB39B's role in membrane traffic, autophagy, and associated neuropathology.J Cell Physiol. 2021 Mar;236(3):1579-1592. doi: 10.1002/jcp.29962. Epub 2020 Aug 6. J Cell Physiol. 2021. PMID: 32761840 Review.
-
Dysfunction of RAB39B-Mediated Vesicular Trafficking in Lewy Body Diseases.Mov Disord. 2021 Aug;36(8):1744-1758. doi: 10.1002/mds.28605. Epub 2021 May 3. Mov Disord. 2021. PMID: 33939203 Review.
Cited by
-
Rab family of GTPases.Methods Mol Biol. 2015;1298:1-15. doi: 10.1007/978-1-4939-2569-8_1. Methods Mol Biol. 2015. PMID: 25800828 Free PMC article. Review.
-
A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy.Sci Adv. 2016 Sep 2;2(9):e1601167. doi: 10.1126/sciadv.1601167. eCollection 2016 Sep. Sci Adv. 2016. PMID: 27617292 Free PMC article.
-
CCDC22: a novel candidate gene for syndromic X-linked intellectual disability.Mol Psychiatry. 2012 Jan;17(1):4-7. doi: 10.1038/mp.2011.95. Epub 2011 Aug 9. Mol Psychiatry. 2012. PMID: 21826058 Free PMC article. No abstract available.
-
Consequences of Rab GTPase dysfunction in genetic or acquired human diseases.Small GTPases. 2018 Mar 4;9(1-2):158-181. doi: 10.1080/21541248.2017.1397833. Epub 2017 Dec 28. Small GTPases. 2018. PMID: 29239692 Free PMC article. Review.
-
Mechanistic target of rapamycin signaling in human nervous system development and disease.Front Mol Neurosci. 2022 Sep 26;15:1005631. doi: 10.3389/fnmol.2022.1005631. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36226315 Free PMC article. Review.
References
-
- Herbst D.S. Nonspecific X-linked mental retardation I: A review with information from 24 new families. Am. J. Med. Genet. 1980;7:443–460. - PubMed
-
- D'Adamo P., Menegon A., Lo Nigro C., Grasso M., Gulisano M., Tamanini F., Bienvenu T., Gedeon A.K., Oostra B., Wu S.K. Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat. Genet. 1998;19:134–139. - PubMed
-
- D'Adamo P., Welzl H., Papadimitriou S., Raffaele di Barletta M., Tiveron C., Tatangelo L., Pozzi L., Chapman P.F., Knevett S.G., Ramsay M.F. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum. Mol. Genet. 2002;11:2567–2580. - PubMed
-
- Bianchi V., Farisello P., Baldelli P., Meskenaite V., Milanese M., Vecellio M., Muhlemann S., Lipp H.P., Bonanno G., Benfenati F. Cognitive impairment in Gdi1-deficient mice is associated with altered synaptic vesicle pools and short-term synaptic plasticity, and can be corrected by appropriate learning training. Hum. Mol. Genet. 2009;18:105–117. - PMC - PubMed
-
- Pereira-Leal J.B., Seabra M.C. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000;301:1077–1087. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

