Disorganized innervation and neuronal loss in the inner ear of Slitrk6-deficient mice
- PMID: 19936227
- PMCID: PMC2777407
- DOI: 10.1371/journal.pone.0007786
Disorganized innervation and neuronal loss in the inner ear of Slitrk6-deficient mice
Abstract
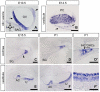
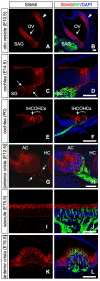
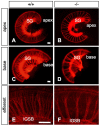
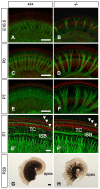
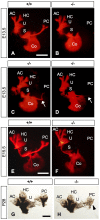
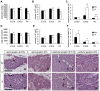
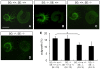
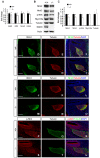
Slitrks are type I transmembrane proteins that share conserved leucine-rich repeat domains similar to those in the secreted axonal guidance molecule Slit. They also show similarities to Ntrk neurotrophin receptors in their carboxy-termini, sharing a conserved tyrosine residue. Among 6 Slitrk family genes in mammals, Slitrk6 has a unique expression pattern, with strong expression in the sensory epithelia of the inner ear. We generated Slitrk6-knockout mice and investigated the development of their auditory and vestibular sensory organs. Slitrk6-deficient mice showed pronounced reduction in the cochlear innervation. In the vestibule, the innervation to the posterior crista was often lost, reduced, or sometimes misguided. These defects were accompanied by the loss of neurons in the spiral and vestibular ganglia. Cochlear sensory epithelia from Slitrk6-knockout mice have reduced ability in promoting neurite outgrowth of spiral ganglion neurons. Indeed the Slitrk6-deficient inner ear showed a mild but significant decrease in the expression of Bdnf and Ntf3, both of which are essential for the innervation and survival of sensory neurons. In addition, the expression of Ntrk receptors, including their phosphorylated forms was decreased in Slitrk6-knockout cochlea. These results suggest that Slitrk6 promotes innervation and survival of inner ear sensory neurons by regulating the expression of trophic and/or tropic factors including neurotrophins from sensory epithelia.
Conflict of interest statement
Figures

Similar articles
-
Brn3c null mutant mice show long-term, incomplete retention of some afferent inner ear innervation.BMC Neurosci. 2003 Jan 30;4:2. doi: 10.1186/1471-2202-4-2. BMC Neurosci. 2003. PMID: 12585968 Free PMC article.
-
Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain.Hear Res. 2005 Aug;206(1-2):52-63. doi: 10.1016/j.heares.2004.11.025. Hear Res. 2005. PMID: 16080998 Free PMC article. Review.
-
Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance.Prog Brain Res. 2004;146:265-78. doi: 10.1016/S0079-6123(03)46017-2. Prog Brain Res. 2004. PMID: 14699969 Review.
-
Neurod1 regulates survival and formation of connections in mouse ear and brain.Cell Tissue Res. 2010 Jul;341(1):95-110. doi: 10.1007/s00441-010-0984-6. Epub 2010 May 30. Cell Tissue Res. 2010. PMID: 20512592 Free PMC article.
-
Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation.Hear Res. 1994 May;75(1-2):131-44. doi: 10.1016/0378-5955(94)90064-7. Hear Res. 1994. PMID: 8071140
Cited by
-
SLITRK6 mutations cause myopia and deafness in humans and mice.J Clin Invest. 2013 May;123(5):2094-102. doi: 10.1172/JCI65853. Epub 2013 Apr 1. J Clin Invest. 2013. PMID: 23543054 Free PMC article.
-
ERK Regulates NeuroD1-mediated Neurite Outgrowth via Proteasomal Degradation.Exp Neurobiol. 2020 Jun 30;29(3):189-206. doi: 10.5607/en20021. Exp Neurobiol. 2020. PMID: 32606250 Free PMC article.
-
SLITRK5 is a negative regulator of hedgehog signaling in osteoblasts.Nat Commun. 2021 Jul 29;12(1):4611. doi: 10.1038/s41467-021-24819-w. Nat Commun. 2021. PMID: 34326333 Free PMC article.
-
Insight into the Association between Slitrk Protein and Neurodevelopmental and Neuropsychiatric Conditions.Biomolecules. 2024 Aug 26;14(9):1060. doi: 10.3390/biom14091060. Biomolecules. 2024. PMID: 39334827 Free PMC article. Review.
-
Developmental control of noradrenergic system by SLITRK1 and its implications in the pathophysiology of neuropsychiatric disorders.Front Mol Neurosci. 2023 Jan 4;15:1080739. doi: 10.3389/fnmol.2022.1080739. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36683853 Free PMC article. Review.
References
-
- Aruga J, Mikoshiba K. Identification and characterization of Slitrk, a novel neuronal transmembrane protein family controlling neurite outgrowth. Mol Cell Neurosci. 2003;24:117–129. - PubMed
-
- Aruga J, Yokota N, Mikoshiba K. Human SLITRK family genes: genomic organization and expression profiling in normal brain and brain tumor tissue. Gene. 2003;315:87–94. - PubMed
-
- Kobe B, Kajava AV. The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol. 2001;11:725–732. - PubMed
-
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000;10:95–102. - PubMed
-
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

