Mitochondrial neurogastrointestinal encephalopathy due to mutations in RRM2B
- PMID: 19667227
- PMCID: PMC2747647
- DOI: 10.1001/archneurol.2009.139
Mitochondrial neurogastrointestinal encephalopathy due to mutations in RRM2B
Abstract
Background: Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is a progressive neurodegenerative disorder associated with thymidine phosphorylase deficiency resulting in high levels of plasma thymidine and a characteristic clinical phenotype.
Objective: To investigate the molecular basis of MNGIE in a patient with a normal plasma thymidine level.
Design: Clinical, neurophysiological, and histopathological examinations as well as molecular and genetic analyses.
Setting: Nerve and muscle center and genetic clinic. Patient A 42-year-old woman with clinical findings strongly suggestive for MNGIE.
Main outcome measures: Clinical description of the disease and its novel genetic cause.
Results: Identification of mitochondrial DNA depletion in muscle samples (approximately 12% of the control mean content) prompted us to look for other causes of our patient's condition. Sequencing of genes associated with mitochondrial DNA depletion-POLG, PEO1, ANT1, SUCLG1, and SUCLA2-did not reveal deleterious mutations. Results of sequencing and array comparative genomic hybridization of the mitochondrial DNA for point mutations and deletions in blood and muscle were negative. Sequencing of RRM2B, a gene encoding cytosolic p53-inducible ribonucleoside reductase small subunit (RIR2B), revealed 2 pathogenic mutations, c.329G>A (p.R110H) and c.362G>A (p.R121H). These mutations are predicted to affect the docking interface of the RIR2B homodimer and likely result in impaired enzyme activity.
Conclusions: This study expands the clinical spectrum of impaired RIR2B function, challenges the notion of locus homogeneity of MNGIE, and sheds light on the pathogenesis of conditions involved in the homeostasis of the mitochondrial nucleotide pool. Our findings suggest that patients with MNGIE who have normal thymidine levels should be tested for RRM2B mutations.
Figures
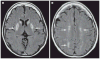

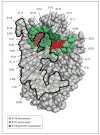
Comment in
-
Mitochondrial neurogastrointestinal encephalopathy without elevated thymidine levels.Arch Neurol. 2010 May;67(5):644; author reply 644-5. doi: 10.1001/archneurol.2010.73. Arch Neurol. 2010. PMID: 20457971 No abstract available.
Similar articles
-
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)-like phenotype: an expanded clinical spectrum of POLG1 mutations.J Neurol. 2012 May;259(5):862-8. doi: 10.1007/s00415-011-6268-6. Epub 2011 Oct 13. J Neurol. 2012. PMID: 21993618
-
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): a disease of two genomes.Neurologist. 2004 Jan;10(1):8-17. doi: 10.1097/01.nrl.0000106919.06469.04. Neurologist. 2004. PMID: 14720311 Review.
-
Mitochondrial DNA depletion syndrome due to mutations in the RRM2B gene.Neuromuscul Disord. 2008 Jun;18(6):453-9. doi: 10.1016/j.nmd.2008.04.006. Epub 2008 May 27. Neuromuscul Disord. 2008. PMID: 18504129 Free PMC article.
-
Mitochondrial neurogastrointestinal encephalomyopathy: evidence of mitochondrial DNA depletion in the small intestine.Gastroenterology. 2006 Mar;130(3):893-901. doi: 10.1053/j.gastro.2006.01.004. Gastroenterology. 2006. PMID: 16530527
-
MNGIE: from nuclear DNA to mitochondrial DNA.Neuromuscul Disord. 2001 Jan;11(1):7-10. doi: 10.1016/s0960-8966(00)00159-0. Neuromuscul Disord. 2001. PMID: 11166160 Review.
Cited by
-
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE)-like phenotype: an expanded clinical spectrum of POLG1 mutations.J Neurol. 2012 May;259(5):862-8. doi: 10.1007/s00415-011-6268-6. Epub 2011 Oct 13. J Neurol. 2012. PMID: 21993618
-
Highly mutagenic and severely imbalanced dNTP pools can escape detection by the S-phase checkpoint.Nucleic Acids Res. 2010 Jul;38(12):3975-83. doi: 10.1093/nar/gkq128. Epub 2010 Mar 9. Nucleic Acids Res. 2010. PMID: 20215435 Free PMC article.
-
Enteric Neuromyopathies: Highlights on Genetic Mechanisms Underlying Chronic Intestinal Pseudo-Obstruction.Biomolecules. 2022 Dec 10;12(12):1849. doi: 10.3390/biom12121849. Biomolecules. 2022. PMID: 36551277 Free PMC article. Review.
-
The role of brain MRI in mitochondrial neurogastrointestinal encephalomyopathy.Neuroradiol J. 2013 Oct;26(5):520-30. doi: 10.1177/197140091302600505. Epub 2013 Nov 7. Neuroradiol J. 2013. PMID: 24199812 Free PMC article.
-
Syndromes associated with mitochondrial DNA depletion.Ital J Pediatr. 2014 Apr 3;40:34. doi: 10.1186/1824-7288-40-34. Ital J Pediatr. 2014. PMID: 24708634 Free PMC article. Review.
References
-
- Nishino I, Spinazzola A, Papadimitriou A, et al. Mitochondrial neurogastrointestinal encephalomyopathy: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol. 2000;47(6):792–800. - PubMed
-
- Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283(5402):689–692. - PubMed
-
- Lehnhardt F-G, Horvath R, Ullrich R, et al. Altered cerebral glucose metabolism in a family with clinical features resembling mitochondrial neurogastrointestinal encephalomyopathy syndrome in association with multiple mitochondrial DNA deletions. Arch Neurol. 2008;65(3):407–411. - PubMed
-
- Van Goethem G, Schwartz M, Löfgren A, Dermaut B, Van Broeckhoven C, Vissing J. Novel POLG mutations in progressive external ophthalmoplegia mimicking mitochondrial neurogastrointestinal encephalomyopathy. Eur J Hum Genet. 2003;11(7):547–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

