CTCF: master weaver of the genome
- PMID: 19563753
- PMCID: PMC3040116
- DOI: 10.1016/j.cell.2009.06.001
CTCF: master weaver of the genome
Abstract
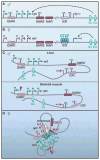
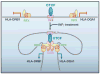
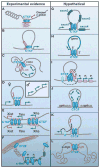
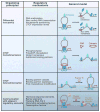
CTCF is a highly conserved zinc finger protein implicated in diverse regulatory functions, including transcriptional activation/repression, insulation, imprinting, and X chromosome inactivation. Here we re-evaluate data supporting these roles in the context of mechanistic insights provided by recent genome-wide studies and highlight evidence for CTCF-mediated intra- and interchromosomal contacts at several developmentally regulated genomic loci. These analyses support a primary role for CTCF in the global organization of chromatin architecture and suggest that CTCF may be a heritable component of an epigenetic system regulating the interplay between DNA methylation, higher-order chromatin structure, and lineage-specific gene expression.
Figures


Similar articles
-
Ribosomal RNA gene transcription mediated by the master genome regulator protein CCCTC-binding factor (CTCF) is negatively regulated by the condensin complex.J Biol Chem. 2013 Sep 6;288(36):26067-26077. doi: 10.1074/jbc.M113.486175. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884423 Free PMC article.
-
Epstein-Barr Virus Rta-Mediated Accumulation of DNA Methylation Interferes with CTCF Binding in both Host and Viral Genomes.J Virol. 2017 Jul 12;91(15):e00736-17. doi: 10.1128/JVI.00736-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28490592 Free PMC article.
-
Transforming growth factor beta promotes complexes between Smad proteins and the CCCTC-binding factor on the H19 imprinting control region chromatin.J Biol Chem. 2010 Jun 25;285(26):19727-37. doi: 10.1074/jbc.M109.088385. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427289 Free PMC article.
-
Involvement of CCCTC-binding factor in epigenetic regulation of cancer.Mol Biol Rep. 2023 Dec;50(12):10383-10398. doi: 10.1007/s11033-023-08879-3. Epub 2023 Oct 15. Mol Biol Rep. 2023. PMID: 37840067 Review.
-
CTCF shapes chromatin by multiple mechanisms: the impact of 20 years of CTCF research on understanding the workings of chromatin.Chromosoma. 2010 Aug;119(4):351-60. doi: 10.1007/s00412-010-0262-0. Epub 2010 Feb 20. Chromosoma. 2010. PMID: 20174815 Free PMC article. Review.
Cited by
-
A subset of Drosophila Myc sites remain associated with mitotic chromosomes colocalized with insulator proteins.Nat Commun. 2013;4:1464. doi: 10.1038/ncomms2469. Nat Commun. 2013. PMID: 23403565 Free PMC article.
-
Genetic determinants of haemolysis in sickle cell anaemia.Br J Haematol. 2013 Apr;161(2):270-8. doi: 10.1111/bjh.12245. Epub 2013 Feb 14. Br J Haematol. 2013. PMID: 23406172 Free PMC article. Clinical Trial.
-
Super-Enhancers and CTCF in Early Embryonic Cell Fate Decisions.Front Cell Dev Biol. 2021 Mar 25;9:653669. doi: 10.3389/fcell.2021.653669. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33842482 Free PMC article. Review.
-
Senescence Mediated by p16INK4a Impedes Reprogramming of Human Corneal Endothelial Cells into Neural Crest Progenitors.Sci Rep. 2016 Oct 14;6:35166. doi: 10.1038/srep35166. Sci Rep. 2016. PMID: 27739458 Free PMC article.
-
Unravelling global genome organization by 3C-seq.Semin Cell Dev Biol. 2012 Apr;23(2):213-21. doi: 10.1016/j.semcdb.2011.11.003. Epub 2011 Nov 18. Semin Cell Dev Biol. 2012. PMID: 22120510 Free PMC article. Review.
References
-
- Apostolou E, Thanos D. Virus Infection Induces NF-kappaB-dependent interchromosomal associations mediating monoallelic IFN-beta gene expression. Cell. 2008;134:85–96. - PubMed
-
- Bacher CP, Guggiari M, Brors B, Augui S, Clerc P, Avner P, Eils R, Heard E. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat Cell Biol. 2006;8:293–299. - PubMed
-
- Baniahmad A, Steiner C, Kohne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–514. - PubMed
-
- Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. - PubMed
-
- Bell AC, Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

