A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia
- PMID: 19557861
- PMCID: PMC4103031
- DOI: 10.1002/ana.21678
A novel Nav1.7 mutation producing carbamazepine-responsive erythromelalgia
Abstract
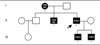
Objective: Human and animal studies have shown that Na(v)1.7 sodium channels, which are preferentially expressed within nociceptors and sympathetic neurons, play a major role in inflammatory and neuropathic pain. Inherited erythromelalgia (IEM) has been linked to gain-of-function mutations of Na(v)1.7. We now report a novel mutation (V400M) in a three-generation Canadian family in which pain is relieved by carbamazepine (CBZ).
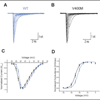
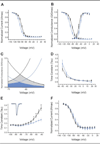
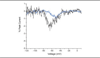
Methods: We extracted genomic DNA from blood samples of eight members of the family, and the sequence of SCN9A coding exons was compared with the reference Na(v)1.7 complementary DNA. Wild-type Na(v)1.7 and V400M cell lines were then analyzed using whole-cell patch-clamp recording for changes in activation, deactivation, steady-state inactivation, and ramp currents.
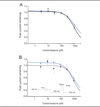
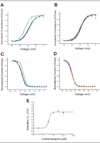
Results: Whole-cell patch-clamp studies of V400M demonstrate changes in activation, deactivation, steady-state inactivation, and ramp currents that can produce dorsal root ganglia neuron hyperexcitability that underlies pain in these patients. We show that CBZ, at concentrations in the human therapeutic range, normalizes the voltage dependence of activation and inactivation of this inherited erythromelalgia mutation in Na(v)1.7 but does not affect these parameters in wild-type Na(v)1.7.
Interpretation: Our results demonstrate a normalizing effect of CBZ on mutant Na(v)1.7 channels in this kindred with CBZ-responsive inherited erythromelalgia. The selective effect of CBZ on the mutant Na(v)1.7 channel appears to explain the ameliorative response to treatment in this kindred. Our results suggest that functional expression and pharmacological studies may provide mechanistic insights into hereditary painful disorders.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures
Similar articles
-
Deletion mutation of sodium channel Na(V)1.7 in inherited erythromelalgia: enhanced slow inactivation modulates dorsal root ganglion neuron hyperexcitability.Brain. 2011 Jul;134(Pt 7):1972-86. doi: 10.1093/brain/awr143. Brain. 2011. PMID: 21705421
-
NaV1.7 gain-of-function mutations as a continuum: A1632E displays physiological changes associated with erythromelalgia and paroxysmal extreme pain disorder mutations and produces symptoms of both disorders.J Neurosci. 2008 Oct 22;28(43):11079-88. doi: 10.1523/JNEUROSCI.3443-08.2008. J Neurosci. 2008. PMID: 18945915 Free PMC article.
-
Na(V)1.7 mutant A863P in erythromelalgia: effects of altered activation and steady-state inactivation on excitability of nociceptive dorsal root ganglion neurons.J Neurosci. 2006 Nov 29;26(48):12566-75. doi: 10.1523/JNEUROSCI.3424-06.2006. J Neurosci. 2006. PMID: 17135418 Free PMC article.
-
Pain disorders and erythromelalgia caused by voltage-gated sodium channel mutations.Curr Neurol Neurosci Rep. 2012 Feb;12(1):76-83. doi: 10.1007/s11910-011-0233-8. Curr Neurol Neurosci Rep. 2012. PMID: 21984269 Review.
-
Familial pain syndromes from mutations of the NaV1.7 sodium channel.Ann N Y Acad Sci. 2010 Jan;1184:196-207. doi: 10.1111/j.1749-6632.2009.05110.x. Ann N Y Acad Sci. 2010. PMID: 20146699 Review.
Cited by
-
A case of sporadic erythromelalgia presenting with small fibre neuropathy.BMJ Case Rep. 2019 Oct 9;12(10):e230549. doi: 10.1136/bcr-2019-230549. BMJ Case Rep. 2019. PMID: 31601551 Free PMC article.
-
Development of a Rapid Throughput Assay for Identification of hNav1.7 Antagonist Using Unique Efficacious Sodium Channel Agonist, Antillatoxin.Mar Drugs. 2016 Feb 16;14(2):36. doi: 10.3390/md14020036. Mar Drugs. 2016. PMID: 26891306 Free PMC article.
-
Between fire and ice: refractory hypothermia and warmth-induced pain in inherited erythromelalgia.BMJ Case Rep. 2017 Jul 26;2017:bcr2017219486. doi: 10.1136/bcr-2017-219486. BMJ Case Rep. 2017. PMID: 28751508 Free PMC article.
-
Neuropathy following spinal nerve injury shares features with the irritable nociceptor phenotype: A back-translational study of oxcarbazepine.Eur J Pain. 2019 Jan;23(1):183-197. doi: 10.1002/ejp.1300. Epub 2018 Aug 28. Eur J Pain. 2019. PMID: 30091265 Free PMC article.
-
Trigeminal neuralgia: An overview from pathophysiology to pharmacological treatments.Mol Pain. 2020 Jan-Dec;16:1744806920901890. doi: 10.1177/1744806920901890. Mol Pain. 2020. PMID: 31908187 Free PMC article. Review.
References
-
- Waxman SG. Neurobiology: a channel sets the gain on pain. Nature. 2006;444:831–832. - PubMed
-
- Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. From genes to pain: Nav 1.7 and human pain disorders. Trends Neurosci. 2007;30:555–563. - PubMed
-
- Black JA, Dib-Hajj S, McNabola K, et al. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res Mol Brain Res. 1996;43:117–131. - PubMed
-
- Sangameswaran L, Fish LM, Koch BD, et al. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J Biol Chem. 1997;272:14805–14809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

