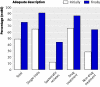
What is missing from descriptions of treatment in trials and reviews?
- PMID: 18583680
- PMCID: PMC2440840
- DOI: 10.1136/bmj.39590.732037.47
What is missing from descriptions of treatment in trials and reviews?
Abstract
Replicating non-pharmacological treatments in practice depends on how well they have been described in research studies
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Ensuring inclusion of research reports in systematic reviews.Arch Phys Med Rehabil. 2009 Nov;90(11 Suppl):S60-9. doi: 10.1016/j.apmr.2009.04.026. Arch Phys Med Rehabil. 2009. PMID: 19892076
-
Challenges in systematic reviews of therapeutic devices and procedures.Ann Intern Med. 2005 Jun 21;142(12 Pt 2):1100-11. doi: 10.7326/0003-4819-142-12_part_2-200506211-00010. Ann Intern Med. 2005. PMID: 15968035 Review.
-
Evaluation of the evidence.Vet Clin North Am Small Anim Pract. 2007 May;37(3):447-62. doi: 10.1016/j.cvsm.2007.01.004. Vet Clin North Am Small Anim Pract. 2007. PMID: 17466749 Review.
-
The Tamiflu trials.BMJ. 2014 Apr 9;348:g2630. doi: 10.1136/bmj.g2630. BMJ. 2014. PMID: 24811414 No abstract available.
-
Tissue viability.J Tissue Viability. 1998 Oct;8(4):2. J Tissue Viability. 1998. PMID: 10480964 No abstract available.
Cited by
-
SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials.BMJ. 2013 Jan 8;346:e7586. doi: 10.1136/bmj.e7586. BMJ. 2013. PMID: 23303884 Free PMC article.
-
A scoping review of classification schemes of interventions to promote and integrate evidence into practice in healthcare.Implement Sci. 2015 Mar 3;10:27. doi: 10.1186/s13012-015-0220-6. Implement Sci. 2015. PMID: 25885047 Free PMC article.
-
Using education to improve control of asthma in children.CMAJ. 2009 Sep 1;181(5):248-9. doi: 10.1503/cmaj.091120. Epub 2009 Aug 17. CMAJ. 2009. PMID: 19687100 Free PMC article. No abstract available.
-
Development of a framework for reporting health service models for managing rheumatoid arthritis.Clin Rheumatol. 2010 Feb;29(2):151-65. doi: 10.1007/s10067-009-1298-5. Epub 2009 Oct 29. Clin Rheumatol. 2010. PMID: 19865842 Free PMC article.
-
Effects of Setting on Psychedelic Experiences, Therapies, and Outcomes: A Rapid Scoping Review of the Literature.Curr Top Behav Neurosci. 2022;56:35-70. doi: 10.1007/7854_2021_298. Curr Top Behav Neurosci. 2022. PMID: 35138585
References
-
- Plint AC, Moher D, Morrison A, Schulz K, Altman DG, Hill C, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 2006;185:263-7. - PubMed
-
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. - PubMed
-
- Dopson S, Locock L, Chambers D, Gabbay J. Implementation of evidence-based medicine: evaluation of the promoting action on clinical effectiveness programme. J Health Serv Res Policy 2006;6:23-31. - PubMed
-
- Pathman DE, Konrad TR, Freed GL, Freeman VA, Koch GG. The awareness-to-adherence model of the steps to clinical guideline compliance. The case of pediatric vaccine recommendations. Med Care 1996;34:873-89. - PubMed
-
- Glasziou P, Haynes B. The paths from research to improved health outcomes. ACP J Club 2005;142:A8-10. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical