A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana
- PMID: 17909185
- PMCID: PMC2000425
- DOI: 10.1073/pnas.0707851104
A chloroplast cyclophilin functions in the assembly and maintenance of photosystem II in Arabidopsis thaliana
Abstract
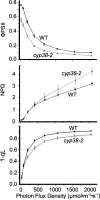
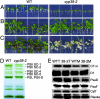
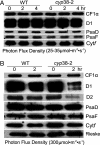
Photosynthetic light reactions rely on the proper function of large protein complexes (including photosystems I and II) that reside in the thylakoid membrane. Although their composition, structure, and function are known, the repertoire of assembly and maintenance factors is still being determined. Here we show that an immunophilin of the cyclophilin type, CYP38, plays a critical role in the assembly and maintenance of photosystem II (PSII) supercomplexes (SCs) in Arabidopsis. Mutant plants with the CYP38 gene interrupted by T-DNA insertion showed stunted growth and were hypersensitive to high light. Leaf chlorophyll fluorescence analysis and thylakoid membrane composition indicated that cyp38 mutant plants had defects in PSII SCs. Sucrose supplementation enabled the rescue of the mutant phenotype under low-light conditions, but failed to mitigate hypersensitivity to high-light stress. Protein radiolabeling assays showed that, although individual thylakoid proteins were synthesized equally in mutant and wild type, the assembly of the PSII SC was impaired in the mutant. In addition, the D1 and D2 components of the mutant PSII had a short half-life under high-light stress. The results provide evidence that CYP38 is necessary for the assembly and stabilization of PSII.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

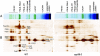


Similar articles
-
Characterization of CYCLOPHILLIN38 shows that a photosynthesis-derived systemic signal controls lateral root emergence.Plant Physiol. 2021 Mar 15;185(2):503-518. doi: 10.1093/plphys/kiaa032. Plant Physiol. 2021. PMID: 33721893 Free PMC article.
-
Complex lumenal immunophilin AtCYP38 influences thylakoid remodelling in Arabidopsis thaliana.J Plant Physiol. 2019 Dec;243:153048. doi: 10.1016/j.jplph.2019.153048. Epub 2019 Oct 1. J Plant Physiol. 2019. PMID: 31639536
-
AtCYP38 ensures early biogenesis, correct assembly and sustenance of photosystem II.Plant J. 2008 Aug;55(4):639-51. doi: 10.1111/j.1365-313X.2008.03532.x. Epub 2008 Apr 24. Plant J. 2008. PMID: 18445132
-
SNOWY COTYLEDON 2 Promotes Chloroplast Development and Has a Role in Leaf Variegation in Both Lotus japonicus and Arabidopsis thaliana.Mol Plant. 2017 May 1;10(5):721-734. doi: 10.1016/j.molp.2017.02.009. Epub 2017 Mar 10. Mol Plant. 2017. PMID: 28286296
-
Small chloroplast-targeted DnaJ proteins are involved in optimization of photosynthetic reactions in Arabidopsis thaliana.BMC Plant Biol. 2010 Mar 7;10:43. doi: 10.1186/1471-2229-10-43. BMC Plant Biol. 2010. PMID: 20205940 Free PMC article.
Cited by
-
Pale-green phenotype of atl31atl6 double mutant leaves is caused by disruption of 5-aminolevulinic acid biosynthesis in Arabidopsis thaliana.PLoS One. 2015 Feb 23;10(2):e0117662. doi: 10.1371/journal.pone.0117662. eCollection 2015. PLoS One. 2015. PMID: 25706562 Free PMC article.
-
Validation of housekeeping genes for gene expression studies in Symbiodinium exposed to thermal and light stress.Mar Biotechnol (NY). 2011 Jun;13(3):355-65. doi: 10.1007/s10126-010-9308-9. Epub 2010 Jul 29. Mar Biotechnol (NY). 2011. PMID: 20668900
-
PsbN is required for assembly of the photosystem II reaction center in Nicotiana tabacum.Plant Cell. 2014 Mar;26(3):1183-99. doi: 10.1105/tpc.113.120444. Epub 2014 Mar 11. Plant Cell. 2014. PMID: 24619613 Free PMC article.
-
Bioinformatic and expression analysis of the Brassica napus L. cyclophilins.Sci Rep. 2017 May 4;7(1):1514. doi: 10.1038/s41598-017-01596-5. Sci Rep. 2017. PMID: 28473712 Free PMC article.
-
A Thylakoid Membrane Protein Functions Synergistically with GUN5 in Chlorophyll Biosynthesis.Plant Commun. 2020 Jul 3;1(5):100094. doi: 10.1016/j.xplc.2020.100094. eCollection 2020 Sep 14. Plant Commun. 2020. PMID: 33367259 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

