Grey literature in meta-analyses of randomized trials of health care interventions
- PMID: 17443631
- PMCID: PMC8973936
- DOI: 10.1002/14651858.MR000010.pub3
Grey literature in meta-analyses of randomized trials of health care interventions
Abstract
Background: The inclusion of grey literature (i.e. literature that has not been formally published) in systematic reviews may help to overcome some of the problems of publication bias, which can arise due to the selective availability of data.
Objectives: To review systematically research studies, which have investigated the impact of grey literature in meta-analyses of randomized trials of health care interventions.
Search strategy: We searched the Cochrane Methodology Register (The Cochrane Library Issue 3, 2005), MEDLINE (1966 to 20 May 2005), the Science Citation Index (June 2005) and contacted researchers who may have carried out relevant studies.
Selection criteria: A study was considered eligible for this review if it compared the effect of the inclusion and exclusion of grey literature on the results of a cohort of meta-analyses of randomized trials.
Data collection and analysis: Data were extracted from each report independently by two reviewers. The main outcome measure was an estimate of the impact of trials from the grey literature on the pooled effect estimates of the meta-analyses. Information was also collected on the area of health care, the number of meta-analyses, the number of trials, the number of trial participants, the year of publication of the trials, the language and country of publication of the trials, the number and type of grey and published literature, and methodological quality.
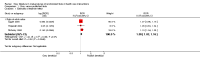
Main results: Five studies met the inclusion criteria. All five studies showed that published trials showed an overall greater treatment effect than grey trials. This difference was statistically significant in one of the five studies. Data could be combined for three of the five studies. This showed that, on average, published trials showed a 9% greater treatment effect than grey trials (ratio of odds ratios for grey versus published trials 1.09; 95% CI 1.03-1.16). Overall there were more published trials included in the meta-analyses than grey trials (median 224 (IQR 108-365) versus 45(IQR 40-102)). Published trials had more participants on average. The most common types of grey literature were abstracts (55%) and unpublished data (30%). There is limited evidence to show whether grey trials are of poorer methodological quality than published trials.
Authors' conclusions: This review shows that published trials tend to be larger and show an overall greater treatment effect than grey trials. This has important implications for reviewers who need to ensure they identify grey trials, in order to minimise the risk of introducing bias into their review.
Conflict of interest statement
Sally Hopewell is the author of one of the studies included in this review and Matthias Egger is author of another of the included studies, which was published as a NHS Health Technology Assessment review.
Figures
Update of
- doi: 10.1002/14651858.MR000010.pub2
Similar articles
-
Bias due to selective inclusion and reporting of outcomes and analyses in systematic reviews of randomised trials of healthcare interventions.Cochrane Database Syst Rev. 2014 Oct 1;2014(10):MR000035. doi: 10.1002/14651858.MR000035.pub2. Cochrane Database Syst Rev. 2014. PMID: 25271098 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research.PLoS One. 2017 Apr 25;12(4):e0176210. doi: 10.1371/journal.pone.0176210. eCollection 2017. PLoS One. 2017. PMID: 28441452 Free PMC article. Review.
-
Selenium supplementation for critically ill adults.Cochrane Database Syst Rev. 2015 Jul 27;2015(7):CD003703. doi: 10.1002/14651858.CD003703.pub3. Cochrane Database Syst Rev. 2015. PMID: 26214143 Free PMC article. Review.
-
A protocol for a systematic review on the impact of unpublished studies and studies published in the gray literature in meta-analyses.Syst Rev. 2013 May 2;2:24. doi: 10.1186/2046-4053-2-24. Syst Rev. 2013. PMID: 23634657 Free PMC article.
Cited by
-
The Use of Antenatal Dexamethasone in Late Preterm and Term Pregnancies to Improve Neonatal Morbidity and Mortality: A Systematic Review and Meta-Analysis.Cureus. 2022 Aug 10;14(8):e27865. doi: 10.7759/cureus.27865. eCollection 2022 Aug. Cureus. 2022. PMID: 36110463 Free PMC article. Review.
-
Mifepristone for uterine fibroids.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD007687. doi: 10.1002/14651858.CD007687.pub2. Cochrane Database Syst Rev. 2012. PMID: 22895965 Free PMC article. Review.
-
Identification of additional trials in prospective trial registers for Cochrane systematic reviews.PLoS One. 2012;7(8):e42812. doi: 10.1371/journal.pone.0042812. Epub 2012 Aug 15. PLoS One. 2012. PMID: 22916163 Free PMC article. Review.
-
Analysis of school support: Systematic literature review of core Chinese- and English-language journals published in 2000-2021.Front Psychol. 2022 Aug 8;13:933695. doi: 10.3389/fpsyg.2022.933695. eCollection 2022. Front Psychol. 2022. PMID: 36003103 Free PMC article.
-
Corticosteroid therapy for nephrotic syndrome in children.Cochrane Database Syst Rev. 2020 Aug 31;2020(8):CD001533. doi: 10.1002/14651858.CD001533.pub6. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2024 Aug 22;8:CD001533. doi: 10.1002/14651858.CD001533.pub7. PMID: 35659203 Free PMC article. Updated. Review.
References
References to studies included in this review
Burdett 2003 {published and unpublished data}
-
- Burdett S, Stewart L. Publication bias and meta‐analysis: a practical example. 8th Cochrane Colloquium: Evidence for Action; 2000 Oct 25‐29; Cape Town, South Africa.
-
- Burdett S, Stewart LA, Tierney JF. Publication bias and meta‐analyses. International Journal of Technology Assessment in Health Care 2003;19(1):129‐34. - PubMed
Egger 2003 {published and unpublished data}
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003;7(1):1‐76. - PubMed
-
- Egger M, Juni P, Bartlett C, Sterne J. Importance of different sources of bias in systematic reviews of controlled trials: a systematic review of empirical studies. 9th Cochrane Colloquium: The evidence dissemination process: how to make it more efficient; 2001 Oct 9‐13; Lyon, France.
Fergusson 2000 {published and unpublished data}
-
- Fergusson D, Laupacis A, Salmi LR, McAlister FA, Huet C. What should be included in meta‐analyses? An exploration of methodological issues using the ISPOT meta‐analyses. International Journal of Technology Assessment in Health Care 2000;16(4):1109‐19. - PubMed
Hopewell 2004 {unpublished data only}
-
- Hopewell S. Impact of grey literature on systematic reviews of randomized trials (D.Phil thesis). Oxford: University of Oxford, 2004.
McAuley 2000 {published data only}
-
- McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta‐analysis. Lancet 2000;356(9237):1228‐31. - PubMed
References to studies excluded from this review
Bhandari 2000 {published data only}
-
- Bhandari M, Guyatt GH, Tong D, Adili A, Shaughnessy SG. Reamed versus nonreamed intramedullary nailing of lower extremity long bone fractures: a systematic overview and meta‐analysis. Journal of Orthopaedic Trauma 2000;14(1):2‐9. - PubMed
Horn 2000 {published data only}
-
- Horn J, Limburg M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2000, Issue 1. - PubMed
Jeng 1995 {published data only}
-
- Jeng GT, Scott JR, Burmeister LF. A comparison of meta‐analytic results using literature vs individual patient data. Paternal cell immunization for recurrent miscarriage. JAMA 1995;274(10):830‐6. - PubMed
Additional references
Alberani 1990
Auger 1998
-
- Auger CP. Information sources in grey literature (guides to information sources). 4th Edition. London: Bowker Saur, 1998.
Chan 2004
-
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. - PubMed
Cook 1993
-
- Cook D, Guyatt GH, Ryan G. Should unpublished data be included in meta‐analyses? Current convictions and controversies. JAMA 1993;269(21):2749‐53. - PubMed
Glass 1981
-
- Glass GV, McGaw B, Smith ML. Meta‐analysis in social research. London: Sage Publication, 1981:65‐8.
Hopewell 2005
-
- Hopewell S, Clarke M. Abstracts presented at the American Society of Clinical Oncology conference: how completely are trials reported?. Clinical Trials 2005;2(3):265‐8. - PubMed
Loo 1985
-
- Loo JV. Medical and psychological effects of unemployment: a 'grey' literature search. Health Libraries Review 1985;2:55‐62.
Mallett 2002
-
- Mallett S, Clarke M. The typical Cochrane review. How many trials? How many participants?. International Journal of Technology Assessment in Health Care 2002;18(4):820‐3. - PubMed
Middleton 2004
-
- Middleton P. How allocation concealment is handled in Cochrane reviews. Chinese Journal of Evidence‐Based Medicine 2004;4(11):711‐3.
Moher 2001
-
- Moher D, Jones A, Lepage L. Use of the CONSORT statement and quality of reports of randomized trials: a comparative before‐and‐after evaluation. JAMA 2001;285(15):1992‐5. - PubMed
Scherer 2007
-
- Scherer RW, Langenberg P, Elm E. Full publication of results initially presented in abstracts. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.MR000005.pub2] - PubMed
Song 2000
-
- Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technology Assessment 2000;4(10):1‐115. - PubMed
Sterne 2000
-
- Sterne J, Bartlett C, Juni P, Egger M. Do we need comprehensive literature searches? A study of publication and language bias in meta‐analyses of controlled trials. 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000 Jul 3‐5; Oxford, UK.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous