Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth
- PMID: 17400654
- PMCID: PMC2576517
- DOI: 10.1093/hmg/ddm060
Loss of MMP-2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth
Abstract
The 'vanishing bone' or inherited osteolysis/arthritis syndromes represent a heterogeneous group of skeletal disorders characterized by mineralization defects of affected bones and joints. Differing in anatomical distribution, severity and associated syndromic features, gene identification in each 'vanishing bone' disorder should provide unique insights into genetic/molecular pathways contributing to the overall control of skeletal growth and development. We previously described and then demonstrated that the novel autosomal recessive osteolysis/arthritis syndrome, multicentric osteolysis with arthritis (MOA) (MIM #605156), was caused by inactivating mutations in the MMP2 gene [Al Aqeel, A., Al Sewairi, W., Edress, B., Gorlin, R.J., Desnick, R.J. and Martignetti, J.A. (2000) Inherited multicentric osteolysis with arthritis: A variant resembling Torg syndrome in a Saudi family. Am. J. Med. Genet., 93, 11-18.]. These in vivo results were counterintuitive and unexpected since previous in vitro studies suggested that MMP-2 overexpression and increased activity, not deficiency, would result in the bone and joint features of MOA. The apparent lack of a murine model [Itoh, T., Ikeda, T., Gomi, H., Nakao, S., Suzuki, T. and Itohara, S. (1997) Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J. Biol. Chem., 272, 22389-22392.] has hindered studies on disease pathogenesis and, more fundamentally, in addressing the paradox of how functional loss of a single proteolytic enzyme results in an apparent increase in bone loss. Here, we report that Mmp2-/- mice display attenuated features of human MOA including progressive loss of bone mineral density, articular cartilage destruction and abnormal long bone and craniofacial development. Moreover, these changes are associated with markedly and developmentally restricted decreases in osteoblast and osteoclast numbers in vivo. Mmp2-/- mice have approximately 50% fewer osteoblasts and osteoclasts than control littermates at 4 days of life but these differences have nearly resolved by 4 weeks of age. In addition, despite normal cell numbers in vivo at 8 weeks of life, Mmp2-/- bone marrow cells are unable to effectively support osteoblast and osteoclast growth and differentiation in culture. Targeted inhibition of MMP-2 using siRNA in human SaOS2 and murine MC3T3 osteoblast cell lines resulted in decreased cell proliferation rates. Taken together, our findings suggest that MMP-2 plays a direct role in early skeletal development and bone cell growth and proliferation. Thus, Mmp2-/- mice provide a valuable biological resource for studying the pathophysiological mechanisms underlying the human disease and defining the in vivo physiological role of MMP-2.
Conflict of interest statement
Figures

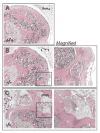
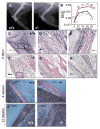
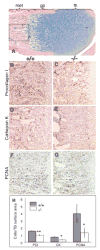
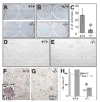
Similar articles
-
Loss of MMP-2 in murine osteoblasts upregulates osteopontin and bone sialoprotein expression in a circuit regulating bone homeostasis.Dis Model Mech. 2013 Mar;6(2):397-403. doi: 10.1242/dmm.007914. Epub 2012 Aug 23. Dis Model Mech. 2013. PMID: 22917927 Free PMC article.
-
Type I collagen is a genetic modifier of matrix metalloproteinase 2 in murine skeletal development.Dev Dyn. 2007 Jun;236(6):1683-93. doi: 10.1002/dvdy.21159. Dev Dyn. 2007. PMID: 17440987 Free PMC article.
-
Osteoclast deficiency results in disorganized matrix, reduced mineralization, and abnormal osteoblast behavior in developing bone.J Bone Miner Res. 2004 Sep;19(9):1441-51. doi: 10.1359/JBMR.040514. Epub 2004 Jun 2. J Bone Miner Res. 2004. PMID: 15312244
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Biological aspects of altered bone remodeling in multiple myeloma and possibilities of pharmacological intervention.Dan Med Bull. 2011 May;58(5):B4277. Dan Med Bull. 2011. PMID: 21535989 Review.
Cited by
-
Association between genetic polymorphisms of MMP8 and the risk of steroid-induced osteonecrosis of the femoral head in the population of northern China.Medicine (Baltimore). 2016 Sep;95(37):e4794. doi: 10.1097/MD.0000000000004794. Medicine (Baltimore). 2016. PMID: 27631232 Free PMC article.
-
Expression of matrix metalloproteinases-2 and -9 and RECK during alveolar bone regeneration in rat.J Mol Histol. 2008 Apr;39(2):201-8. doi: 10.1007/s10735-007-9152-z. Epub 2007 Nov 7. J Mol Histol. 2008. PMID: 17987394
-
Brazilian Multiethnic Association Study of Genetic Variant Interactions among FOS, CASP8, MMP2 and CRISPLD2 in the Risk of Nonsyndromic Cleft Lip with or without Cleft Palate.Dent J (Basel). 2022 Dec 26;11(1):7. doi: 10.3390/dj11010007. Dent J (Basel). 2022. PMID: 36661544 Free PMC article.
-
Heterochronic shift in gene expression leads to ontogenetic morphological divergence between two closely related polyploid species.iScience. 2024 Mar 25;27(4):109566. doi: 10.1016/j.isci.2024.109566. eCollection 2024 Apr 19. iScience. 2024. PMID: 38632992 Free PMC article.
-
The collagen receptor uPARAP/Endo180 in tissue degradation and cancer (Review).Int J Oncol. 2015 Oct;47(4):1177-88. doi: 10.3892/ijo.2015.3120. Epub 2015 Aug 12. Int J Oncol. 2015. PMID: 26316068 Free PMC article. Review.
References
-
- Al Aqeel A, Al Sewairi W, Edress B, Gorlin RJ, Desnick RJ, Martignetti JA. Inherited multicentric osteolysis with arthritis: A variant resembling Torg syndrome in a Saudi family. Am J Med Genet. 2000;93:11–18. - PubMed
-
- Itoh T, Ikeda T, Gomi H, Nakao S, Suzuki T, Itohara S. Unaltered secretion of beta-amyloid precursor protein in gelatinase A (matrix metalloproteinase 2)-deficient mice. J Biol Chem. 1997;272:22389–22392. - PubMed
-
- Torg JS, Steel HH. Essential osteolysis with nephropathy. A review of the literature and case report of an unusual syndrome. J Bone Joint Surg Am. 1968;50:1629–1638. - PubMed
-
- Tyler T, Rosenbaum HD. Idiopathic multicentric osteolysis. AJR Am J Roentgenol. 1976;126:23–31. - PubMed
-
- Jackson J. A boneless arm. Boston Med Surg J. 1838;18:368–369.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

