Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites
- PMID: 17242517
- PMCID: PMC2483498
- DOI: 10.1107/S090744490604947X
Ceruloplasmin revisited: structural and functional roles of various metal cation-binding sites
Abstract
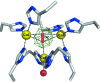
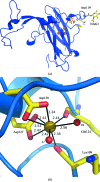
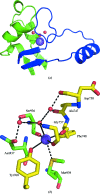
The three-dimensional molecular structure of human serum ceruloplasmin has been reinvestigated using X-ray synchrotron data collected at 100 K from a crystal frozen to liquid-nitrogen temperature. The resulting model, with an increase in resolution from 3.1 to 2.8 A, gives an overall improvement of the molecular structure, in particular the side chains. In addition, it enables the clear definition of previously unidentified Ca2+-binding and Na+-binding sites. The Ca2+ cation is located in domain 1 in a configuration very similar to that found in the activated bovine factor Va. The Na+ sites appear to play a structural role in providing rigidity to the three protuberances on the top surface of the molecule. These features probably help to steer substrates towards the mononuclear copper sites prior to their oxidation and to restrict the size of the approaching substrate. The trinuclear copper centre appears to differ from the room-temperature structure in that a dioxygen moiety is bound in a similar way to that found in the endospore coat protein CotA from Bacillus subtilis.
Figures
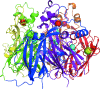
Similar articles
-
An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi-copper oxidase in the plasma.J Biol Inorg Chem. 1999 Oct;4(5):579-87. doi: 10.1007/s007750050380. J Biol Inorg Chem. 1999. PMID: 10550686
-
Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties.J Biol Chem. 2003 May 23;278(21):19416-25. doi: 10.1074/jbc.M301251200. Epub 2003 Mar 13. J Biol Chem. 2003. PMID: 12637519
-
The role of Glu498 in the dioxygen reactivity of CotA-laccase from Bacillus subtilis.Dalton Trans. 2010 Mar 21;39(11):2875-82. doi: 10.1039/b922734b. Epub 2010 Feb 5. Dalton Trans. 2010. PMID: 20200715
-
Role of metal in folding and stability of copper proteins in vitro.Biochim Biophys Acta. 2012 Sep;1823(9):1594-603. doi: 10.1016/j.bbamcr.2012.01.013. Epub 2012 Jan 28. Biochim Biophys Acta. 2012. PMID: 22306006 Review.
-
[Ceruloplasmin, hephaestin and zyklopen: the three multicopper oxidases important for human iron metabolism].Postepy Hig Med Dosw (Online). 2014;68:912-24. doi: 10.5604/17322693.1111136. Postepy Hig Med Dosw (Online). 2014. PMID: 24988611 Review. Polish.
Cited by
-
The interplay of transition metals in ferroptosis and pyroptosis.Cell Div. 2024 Aug 3;19(1):24. doi: 10.1186/s13008-024-00127-9. Cell Div. 2024. PMID: 39097717 Free PMC article. Review.
-
The tertiary structure and domain organization of coagulation factor VIII.Blood. 2008 Feb 1;111(3):1240-7. doi: 10.1182/blood-2007-08-109918. Epub 2007 Oct 26. Blood. 2008. PMID: 17965321 Free PMC article.
-
Serum and brain natural copper stable isotopes in a mouse model of Alzheimer's disease.Sci Rep. 2019 Aug 15;9(1):11894. doi: 10.1038/s41598-019-47790-5. Sci Rep. 2019. PMID: 31417103 Free PMC article.
-
Potential Antioxidant Activity of Calcium and Selected Oxidative Stress Markers in Lead- and Cadmium-Exposed Workers.Oxid Med Cell Longev. 2020 Oct 5;2020:8035631. doi: 10.1155/2020/8035631. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 33082913 Free PMC article.
-
Ceruloplasmin protects injured spinal cord from iron-mediated oxidative damage.J Neurosci. 2008 Nov 26;28(48):12736-47. doi: 10.1523/JNEUROSCI.3649-08.2008. J Neurosci. 2008. PMID: 19036966 Free PMC article.
References
-
- Banci, L., Bertini, I., Cantina, F., DellaMalva, N., Herrmann, T., Rosato, A. & Wuthrich, K. (2006). J. Biol. Chem.281, 29141–29147. - PubMed
-
- Bento, I., Martins, L. O., Lopes, G. G., Carrondo, M. A. & Lindley, P. F. (2005). Dalton Trans.21, 3507–3513. - PubMed
-
- Bielli, P., Bellenchini, G. C. & Calabrese, L. (2001). J. Biol. Chem.276, 2678–2685. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

