Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels
- PMID: 16166153
- PMCID: PMC1464207
- DOI: 10.1113/jphysiol.2005.097220
Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels
Abstract
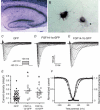
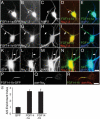
Genetic ablation of the fibroblast growth factor (Fgf) 14 gene in mice or a missense mutation in Fgf14 in humans causes ataxia and cognitive deficits. These phenotypes suggest that the neuronally expressed Fgf14 gene is essential for regulating normal neuronal activity. Here, we demonstrate that FGF14 interacts directly with multiple voltage-gated Na(+) (Nav) channel alpha subunits heterologously expressed in non-neuronal cells or natively expressed in a murine neuroblastoma cell line. Functional studies reveal that these interactions result in the potent inhibition of Nav channel currents (I(Na)) and in changes in the voltage dependence of channel activation and inactivation. Deletion of the unique amino terminus of the splice variant of Fgf14, Fgf14-1b, or expression of the splice variant Fgf14-1a modifies the modulatory effects on I(Na), suggesting an important role for the amino terminus domain of FGF14 in the regulation of Na(v) channels. To investigate the function of FGF14 in neurones, we directly expressed Fgf14 in freshly isolated primary rat hippocampal neurones. In these cells, the addition of FGF14-1a-GFP or FGF14-1b-GFP increased I(Na) density and shifted the voltage dependence of channel activation and inactivation. In fully differentiated neurones, FGF14-1a-GFP or FGF14-1b-GFP preferentially colocalized with endogenous Nav channels at the axonal initial segment, a critical region for action potential generation. Together, these findings implicate FGF14 as a unique modulator of Nav channel activity in the CNS and provide a possible mechanism to explain the neurological phenotypes observed in mice and humans with mutations in Fgf14.
Figures



Similar articles
-
FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels.Mol Cell Neurosci. 2009 Oct;42(2):90-101. doi: 10.1016/j.mcn.2009.05.007. Epub 2009 May 22. Mol Cell Neurosci. 2009. PMID: 19465131 Free PMC article.
-
The FGF14(F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability.J Neurosci. 2007 Oct 31;27(44):12033-44. doi: 10.1523/JNEUROSCI.2282-07.2007. J Neurosci. 2007. PMID: 17978045 Free PMC article.
-
Identification of Amino Acid Residues in Fibroblast Growth Factor 14 (FGF14) Required for Structure-Function Interactions with Voltage-gated Sodium Channel Nav1.6.J Biol Chem. 2016 May 20;291(21):11268-84. doi: 10.1074/jbc.M115.703868. Epub 2016 Mar 18. J Biol Chem. 2016. PMID: 26994141 Free PMC article.
-
Molecular properties of brain sodium channels: an important target for anticonvulsant drugs.Adv Neurol. 1999;79:441-56. Adv Neurol. 1999. PMID: 10514834 Review.
-
Intracellular Fibroblast Growth Factor 14: Emerging Risk Factor for Brain Disorders.Front Cell Neurosci. 2017 Apr 19;11:103. doi: 10.3389/fncel.2017.00103. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28469558 Free PMC article. Review.
Cited by
-
Navigating the intricacies of cellular machinery.J Biol Chem. 2021 Jan-Jun;296:100832. doi: 10.1016/j.jbc.2021.100832. Epub 2021 May 26. J Biol Chem. 2021. PMID: 34048713 Free PMC article.
-
Fibroblast growth factor homologous factors in the heart: a potential locus for cardiac arrhythmias.Trends Cardiovasc Med. 2011 Oct;21(7):199-203. doi: 10.1016/j.tcm.2012.05.010. Trends Cardiovasc Med. 2011. PMID: 22867699 Free PMC article. Review.
-
Genome-Wide Loss of Heterozygosity and DNA Copy Number Aberration in HPV-Negative Oral Squamous Cell Carcinoma and Their Associations with Disease-Specific Survival.PLoS One. 2015 Aug 6;10(8):e0135074. doi: 10.1371/journal.pone.0135074. eCollection 2015. PLoS One. 2015. PMID: 26247464 Free PMC article.
-
TNFR1 signaling converging on FGF14 controls neuronal hyperactivity and sickness behavior in experimental cerebral malaria.J Neuroinflammation. 2023 Dec 19;20(1):306. doi: 10.1186/s12974-023-02992-7. J Neuroinflammation. 2023. PMID: 38115011 Free PMC article.
-
Long-term inactivation particle for voltage-gated sodium channels.J Physiol. 2010 Oct 1;588(Pt 19):3695-711. doi: 10.1113/jphysiol.2010.192559. Epub 2010 Aug 2. J Physiol. 2010. PMID: 20679355 Free PMC article.
References
-
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. - PubMed
-
- Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Molecular mechanism of convergent regulation of brain Na+ channels by protein kinase C and protein kinase A anchored to AKAP-15. Mol Cell Neurosci. 2002;21:63–80. - PubMed
-
- Carpaneto A, Accardi A, Pisciotta M, Gambale F. Chloride channels activated by hypotonicity in N2A neuroblastoma cell line. Exp Brain Res. 1999;124:193–199. - PubMed
-
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. - PubMed
-
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

