Structure of a novel photoreceptor, the BLUF domain of AppA from Rhodobacter sphaeroides
- PMID: 15924418
- PMCID: PMC2774740
- DOI: 10.1021/bi0502691
Structure of a novel photoreceptor, the BLUF domain of AppA from Rhodobacter sphaeroides
Abstract
The flavin-binding BLUF domain of AppA represents a new class of blue light photoreceptors that are present in a number of bacterial and algal species. The dark state X-ray structure of this domain was determined at 2.3 A resolution. The domain demonstrates a new function for the common ferredoxin-like fold; two long alpha-helices flank the flavin, which is bound with its isoalloxazine ring perpendicular to a five-stranded beta-sheet. The hydrogen bond network and the overall protein topology of the BLUF domain (but not its sequence) bear some resemblance to LOV domains, a subset of PAS domains widely involved in signaling. Nearly all residues conserved in BLUF domains surround the flavin chromophore, many of which are involved in an intricate hydrogen bond network. Photoactivation may induce a rearrangement in this network via reorientation of the Gln63 side chain to form a new hydrogen bond to the flavin O4 position. This shift would also break a hydrogen bond to the Trp104 side chain, which may be critical in induction of global structural change in AppA.
Figures
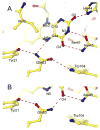
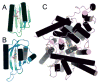
Similar articles
-
Crystal structures of the AppA BLUF domain photoreceptor provide insights into blue light-mediated signal transduction.J Mol Biol. 2006 Sep 29;362(4):717-32. doi: 10.1016/j.jmb.2006.07.024. Epub 2006 Jul 15. J Mol Biol. 2006. PMID: 16949615
-
Light-induced structural changes of apoprotein and chromophore in the sensor of blue light using FAD (BLUF) domain of AppA for a signaling state.Biochemistry. 2005 Feb 1;44(4):1215-24. doi: 10.1021/bi047876t. Biochemistry. 2005. PMID: 15667215
-
The critical role of a hydrogen bond between Gln63 and Trp104 in the blue-light sensing BLUF domain that controls AppA activity.J Mol Biol. 2007 May 18;368(5):1223-30. doi: 10.1016/j.jmb.2007.02.087. Epub 2007 Mar 6. J Mol Biol. 2007. PMID: 17399741
-
BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms.Trends Biochem Sci. 2002 Oct;27(10):497-500. doi: 10.1016/s0968-0004(02)02181-3. Trends Biochem Sci. 2002. PMID: 12368079 Review.
-
Ultrafast spectroscopy of biological photoreceptors.Curr Opin Struct Biol. 2007 Oct;17(5):623-30. doi: 10.1016/j.sbi.2007.09.006. Epub 2007 Oct 23. Curr Opin Struct Biol. 2007. PMID: 17959372 Review.
Cited by
-
Redox modulation of flavin and tyrosine determines photoinduced proton-coupled electron transfer and photoactivation of BLUF photoreceptors.J Biol Chem. 2012 Sep 14;287(38):31725-38. doi: 10.1074/jbc.M112.391896. Epub 2012 Jul 25. J Biol Chem. 2012. PMID: 22833672 Free PMC article.
-
Molecular models predict light-induced glutamine tautomerization in BLUF photoreceptors.Biophys J. 2008 May 15;94(10):3872-9. doi: 10.1529/biophysj.107.124172. Epub 2008 Feb 8. Biophys J. 2008. PMID: 18263659 Free PMC article.
-
Time-resolved spectroscopic studies of the AppA blue-light receptor BLUF domain from Rhodobacter sphaeroides.Biochemistry. 2005 Dec 13;44(49):15978-85. doi: 10.1021/bi050839x. Biochemistry. 2005. PMID: 16331957 Free PMC article.
-
Tripping the light fantastic: blue-light photoreceptors as examples of environmentally modulated protein-protein interactions.Biochemistry. 2011 Jan 11;50(1):4-16. doi: 10.1021/bi101665s. Epub 2010 Dec 14. Biochemistry. 2011. PMID: 21141905 Free PMC article. Review.
-
Molecular eyes: proteins that transform light into biological information.Interface Focus. 2013 Oct 6;3(5):20130005. doi: 10.1098/rsfs.2013.0005. Interface Focus. 2013. PMID: 24511384 Free PMC article. Review.
References
-
- Briggs WR, Huala E. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 1999;15:33–62. - PubMed
-
- Crosson S, Rajagopal S, Moffat K. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 2003;42:2–10. - PubMed
-
- Gomelsky M, Klug G. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci. 2002;27:497–500. - PubMed
-
- Lin C. Plant blue-light receptors. Trends Plant Sci. 2000;5:337–342. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

