Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network
- PMID: 15917292
- PMCID: PMC1182289
- DOI: 10.1091/mbc.e05-02-0120
Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network
Abstract
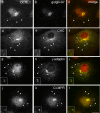
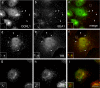
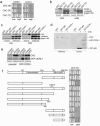
Oculocerebrorenal syndrome of Lowe is caused by mutation of OCRL1, a phosphatidylinositol 4,5-bisphosphate 5-phosphatase localized at the Golgi apparatus. The cellular role of OCRL1 is unknown, and consequently the mechanism by which loss of OCRL1 function leads to disease is ill defined. Here, we show that OCRL1 is associated with clathrin-coated transport intermediates operating between the trans-Golgi network (TGN) and endosomes. OCRL1 interacts directly with clathrin heavy chain and promotes clathrin assembly in vitro. Interaction with clathrin is not, however, required for membrane association of OCRL1. Overexpression of OCRL1 results in redistribution of clathrin and the cation-independent mannose 6-phosphate receptor (CI-MPR) to enlarged endosomal structures that are defective in retrograde trafficking to the TGN. Depletion of cellular OCRL1 also causes partial redistribution of a CI-MPR reporter to early endosomes. These findings suggest a role for OCRL1 in clathrin-mediated trafficking of proteins from endosomes to the TGN and that defects in this pathway might contribute to the Lowe syndrome phenotype.
Figures

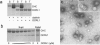

Similar articles
-
OCRL1 engages with the F-BAR protein pacsin 2 to promote biogenesis of membrane-trafficking intermediates.Mol Biol Cell. 2016 Jan 1;27(1):90-107. doi: 10.1091/mbc.E15-06-0329. Epub 2015 Oct 28. Mol Biol Cell. 2016. PMID: 26510499 Free PMC article.
-
The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module.Hum Mol Genet. 2012 Dec 1;21(23):5019-38. doi: 10.1093/hmg/dds343. Epub 2012 Aug 19. Hum Mol Genet. 2012. PMID: 22907655 Free PMC article.
-
Lowe syndrome protein OCRL1 interacts with Rac GTPase in the trans-Golgi network.Hum Mol Genet. 2003 Oct 1;12(19):2449-56. doi: 10.1093/hmg/ddg250. Epub 2003 Jul 29. Hum Mol Genet. 2003. PMID: 12915445
-
Structure and function of the Lowe syndrome protein OCRL1.Traffic. 2005 Sep;6(9):711-9. doi: 10.1111/j.1600-0854.2005.00311.x. Traffic. 2005. PMID: 16101675 Review.
-
The cellular and physiological functions of the Lowe syndrome protein OCRL1.Traffic. 2014 May;15(5):471-87. doi: 10.1111/tra.12160. Epub 2014 Mar 7. Traffic. 2014. PMID: 24499450 Free PMC article. Review.
Cited by
-
Cargo trafficking between endosomes and the trans-Golgi network.Histochem Cell Biol. 2013 Sep;140(3):307-15. doi: 10.1007/s00418-013-1125-6. Epub 2013 Jul 14. Histochem Cell Biol. 2013. PMID: 23851467 Review.
-
Mutations associated with Dent's disease affect gating and voltage dependence of the human anion/proton exchanger ClC-5.Front Physiol. 2015 May 19;6:159. doi: 10.3389/fphys.2015.00159. eCollection 2015. Front Physiol. 2015. PMID: 26042048 Free PMC article.
-
A 3D Renal Proximal Tubule on Chip Model Phenocopies Lowe Syndrome and Dent II Disease Tubulopathy.Int J Mol Sci. 2021 May 19;22(10):5361. doi: 10.3390/ijms22105361. Int J Mol Sci. 2021. PMID: 34069732 Free PMC article.
-
An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway.J Cell Biol. 2005 Aug 15;170(4):607-18. doi: 10.1083/jcb.200505128. J Cell Biol. 2005. PMID: 16103228 Free PMC article.
-
Phosphoinositide phosphatases in cell biology and disease.Prog Lipid Res. 2010 Jul;49(3):201-17. doi: 10.1016/j.plipres.2009.12.001. Epub 2010 Jan 5. Prog Lipid Res. 2010. PMID: 20043944 Free PMC article. Review.
References
-
- Attree, O., Olivos, I. M., Okabe, I., Bailey, L. C., Nelson, D. L., Lewis, R. A., McInnes, R. R., and Nussbaum, R. L. (1992). The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358, 239–242. - PubMed
-
- Cremona, O., et al. (1999). Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179–188. - PubMed
-
- Cullen, P. J., Cozier, G. E., Banting, G., and Mellor, H. (2001). Modular phosphoinositide-binding domains–their role in signalling and membrane trafficking. Curr. Biol. 11, R882–R893. - PubMed
-
- De Matteis, M., Godi, A., and Corda, D. (2002). Phosphoinositides and the Golgi complex. Curr. Opin. Cell Biol. 14, 434–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

