Deficiency in 3'-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1)
- PMID: 15647511
- PMCID: PMC546157
- DOI: 10.1093/nar/gki170
Deficiency in 3'-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1)
Abstract
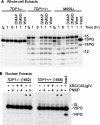
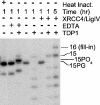
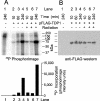
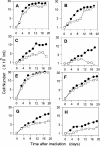
Tyrosyl-DNA phosphodiesterase (TDP1) is a DNA repair enzyme that removes peptide fragments linked through tyrosine to the 3' end of DNA, and can also remove 3'-phosphoglycolates (PGs) formed by free radical-mediated DNA cleavage. To assess whether TDP1 is primarily responsible for PG removal during in vitro end joining of DNA double-strand breaks (DSBs), whole-cell extracts were prepared from lymphoblastoid cells derived either from spinocerebellar ataxia with axonal neuropathy (SCAN1) patients, who have an inactivating mutation in the active site of TDP1, or from closely matched normal controls. Whereas extracts from normal cells catalyzed conversion of 3'-PG termini, both on single-strand oligomers and on 3' overhangs of DSBs, to 3'-phosphate termini, extracts of SCAN1 cells did not process either substrate. Addition of recombinant TDP1 to SCAN1 extracts restored 3'-PG removal, allowing subsequent gap filling on the aligned DSB ends. Two of three SCAN1 lines examined were slightly more radiosensitive than normal cells, but only for fractionated radiation in plateau phase. The results suggest that the TDP1 mutation in SCAN1 abolishes the 3'-PG processing activity of the enzyme, and that there are no other enzymes in cell extracts capable of processing protruding 3'-PG termini. However, the lack of severe radiosensitivity suggests that there must be alternative, TDP1-independent pathways for repair of 3'-PG DSBs.
Figures



Similar articles
-
In vitro complementation of Tdp1 deficiency indicates a stabilized enzyme-DNA adduct from tyrosyl but not glycolate lesions as a consequence of the SCAN1 mutation.DNA Repair (Amst). 2009 May 1;8(5):654-63. doi: 10.1016/j.dnarep.2008.12.012. Epub 2009 Feb 10. DNA Repair (Amst). 2009. PMID: 19211312 Free PMC article.
-
Tyrosyl-DNA phosphodiesterase and the repair of 3'-phosphoglycolate-terminated DNA double-strand breaks.DNA Repair (Amst). 2009 Aug 6;8(8):901-11. doi: 10.1016/j.dnarep.2009.05.003. Epub 2009 Jun 7. DNA Repair (Amst). 2009. PMID: 19505854 Free PMC article.
-
Hereditary ataxia SCAN1 cells are defective for the repair of transcription-dependent topoisomerase I cleavage complexes.DNA Repair (Amst). 2006 Dec 9;5(12):1489-94. doi: 10.1016/j.dnarep.2006.07.004. Epub 2006 Aug 28. DNA Repair (Amst). 2006. PMID: 16935573
-
DNA single-strand break repair and spinocerebellar ataxia with axonal neuropathy-1.Neuroscience. 2007 Apr 14;145(4):1260-6. doi: 10.1016/j.neuroscience.2006.08.048. Epub 2006 Oct 11. Neuroscience. 2007. PMID: 17045754 Review.
-
Spinocerebellar ataxia with axonal neuropathy.Adv Exp Med Biol. 2010;685:75-83. doi: 10.1007/978-1-4419-6448-9_7. Adv Exp Med Biol. 2010. PMID: 20687496 Review.
Cited by
-
Poly(ADP-ribose)polymerase 1 stimulates the AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1.Biosci Rep. 2015 Jun 15;35(4):e00230. doi: 10.1042/BSR20140192. Biosci Rep. 2015. PMID: 26181362 Free PMC article.
-
DNA repair deficiency in neurodegeneration.Prog Neurobiol. 2011 Jul;94(2):166-200. doi: 10.1016/j.pneurobio.2011.04.013. Epub 2011 Apr 30. Prog Neurobiol. 2011. PMID: 21550379 Free PMC article. Review.
-
DNA repair deficiency and neurological disease.Nat Rev Neurosci. 2009 Feb;10(2):100-12. doi: 10.1038/nrn2559. Epub 2009 Jan 15. Nat Rev Neurosci. 2009. PMID: 19145234 Free PMC article. Review.
-
Synthesis and biological evaluation of the first dual tyrosyl-DNA phosphodiesterase I (Tdp1)-topoisomerase I (Top1) inhibitors.J Med Chem. 2012 May 10;55(9):4457-78. doi: 10.1021/jm300335n. Epub 2012 Apr 26. J Med Chem. 2012. PMID: 22536944 Free PMC article.
-
4-Pregnen-21-ol-3,20-dione-21-(4-bromobenzenesulfonate) (NSC 88915) and related novel steroid derivatives as tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors.J Med Chem. 2009 Nov 26;52(22):7122-31. doi: 10.1021/jm901061s. J Med Chem. 2009. PMID: 19883083 Free PMC article.
References
-
- Hsiang Y.H., Hertzberg R., Hecht S., Liu L.F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985;260:14873–14878. - PubMed
-
- Pommier Y. DNA topoisomerase I and II in cancer chemotherapy: update and perspectives. Cancer Chemother. Pharmacol. 1993;32:103–108. - PubMed
-
- Pouliot J.J., Yao K.C., Robertson C.A., Nash H.A. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286:552–555. - PubMed
-
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog. Nucleic Acids Res. Mol. Biol. 1985;32:115–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

