Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival
- PMID: 15371326
- PMCID: PMC522982
- DOI: 10.1101/gad.1239604
Tel/Etv6 is an essential and selective regulator of adult hematopoietic stem cell survival
Abstract
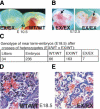
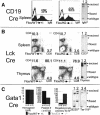
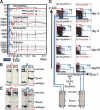
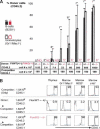
Hematopoietic stem cells (HSCs) sustain blood formation throughout life. Pathways regulating maintenance of adult HSCs are largely unknown. Here we report that the Ets-related transcription factor Tel/Etv6, the product of a locus frequently involved in translocations in leukemia, is a selective regulator of HSC survival. Following inactivation of Tel/Etv6, HSCs are lost in the adult bone marrow but their progeny are unaffected and transiently sustain blood formation. Accordingly, absence of Tel/Etv6 after lineage commitment is ostensibly without consequence except for unexpected impairment of maturation of megakaryocytes. Thus, we establish Tel/Etv6 as a selective and essential regulator of postembryonic HSCs.
Copyright 2004 Cold Spring Harbor Laboratory Press
Figures
Similar articles
-
The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow.Genes Dev. 1998 Aug 1;12(15):2392-402. doi: 10.1101/gad.12.15.2392. Genes Dev. 1998. PMID: 9694803 Free PMC article.
-
Somatic heterozygous mutations in ETV6 (TEL) and frequent absence of ETV6 protein in acute myeloid leukemia.Oncogene. 2005 Jun 9;24(25):4129-37. doi: 10.1038/sj.onc.1208588. Oncogene. 2005. PMID: 15806161
-
Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia.Nat Med. 2005 Jun;11(6):630-7. doi: 10.1038/nm1253. Epub 2005 May 22. Nat Med. 2005. PMID: 15908956
-
ETV6 and ETV7: Siblings in hematopoiesis and its disruption in disease.Crit Rev Oncol Hematol. 2017 Aug;116:106-115. doi: 10.1016/j.critrevonc.2017.05.011. Epub 2017 Jun 3. Crit Rev Oncol Hematol. 2017. PMID: 28693791 Review.
-
ETV6 in hematopoiesis and leukemia predisposition.Semin Hematol. 2017 Apr;54(2):98-104. doi: 10.1053/j.seminhematol.2017.04.005. Epub 2017 Apr 7. Semin Hematol. 2017. PMID: 28637624 Free PMC article. Review.
Cited by
-
Chromatin protein L3MBTL1 is dispensable for development and tumor suppression in mice.J Biol Chem. 2010 Sep 3;285(36):27767-75. doi: 10.1074/jbc.M110.115410. Epub 2010 Jun 30. J Biol Chem. 2010. PMID: 20592034 Free PMC article.
-
Developmental changes in hematopoietic stem cell properties.Exp Mol Med. 2013 Nov 15;45(11):e55. doi: 10.1038/emm.2013.98. Exp Mol Med. 2013. PMID: 24232254 Free PMC article. Review.
-
Chasing Mavericks: The quest for defining developmental waves of hematopoiesis.Curr Top Dev Biol. 2019;132:1-29. doi: 10.1016/bs.ctdb.2019.01.001. Epub 2019 Feb 6. Curr Top Dev Biol. 2019. PMID: 30797507 Free PMC article. Review.
-
Survivin overexpression alone does not alter megakaryocyte ploidy nor interfere with erythroid/megakaryocytic lineage development in transgenic mice.Blood. 2008 Apr 15;111(8):4092-5. doi: 10.1182/blood-2007-11-122150. Epub 2008 Feb 1. Blood. 2008. PMID: 18245663 Free PMC article.
-
Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells.Blood. 2012 Jan 5;119(1):83-94. doi: 10.1182/blood-2011-02-335430. Epub 2011 Nov 16. Blood. 2012. PMID: 22096247 Free PMC article.
References
-
- Brakebusch C., Fillatreau, S., Potocnik, A.J., Bungartz, G., Wilhelm, P., Svensson, M., Kearney, P., Korner, H., Gray, D., and Fassler, R. 2002. β1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity 16: 465-477. - PubMed
-
- Brun A.C., Bjornsson, J.M., Magnusson, M., Larsson, N., Leveen, P., Ehinger, M., Nilsson, E., and Karlsson, S. 2004. Hoxb4 deficient mice have normal hematopoietic development but exhibit a mild proliferation defect in hematopoietic stem cells. Blood. 103(11): 4126-4133. - PubMed
-
- Cheng T., Rodrigues, N., Shen, H., Yang, Y., Dombkowski, D., Sykes, M., and Scadden, D.T. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287: 1804-1808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
