SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression
- PMID: 14701874
- PMCID: PMC305257
- DOI: 10.1101/gad.1153003
SUMO modification of a novel MAR-binding protein, SATB2, modulates immunoglobulin mu gene expression
Abstract
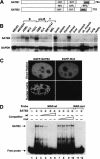
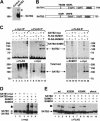
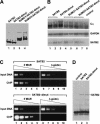
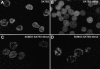
Nuclear matrix attachment regions (MARs) are regulatory DNA sequences that are important for higher-order chromatin organization, long-range enhancer function, and extension of chromatin modifications. Here we characterize a novel cell type-specific MAR-binding protein, SATB2, which binds to the MARs of the endogenous immunoglobulin micro locus in pre-B cells and enhances gene expression. We found that SATB2 differs from the closely related thymocyte-specific protein SATB1 by modifications of two lysines with the small ubiquitive related modifier (SUMO), which are augmented specifically by the SUMO E3 ligase PIAS1. Mutations of the SUMO conjugation sites of SATB2 enhance its activation potential and association with endogenous MARs in vivo, whereas N-terminal fusions with SUMO1 or SUMO3 decrease SATB2-mediated gene activation. Sumoylation is also involved in targeting SATB2 to the nuclear periphery, raising the possibility that this reversible modification of a MAR-binding protein may contribute to the modulation of subnuclear DNA localization.
Figures



Similar articles
-
Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS.Eur J Neurosci. 2005 Feb;21(3):658-68. doi: 10.1111/j.1460-9568.2005.03897.x. Eur J Neurosci. 2005. PMID: 15733084
-
Chromatin loops are selectively anchored using scaffold/matrix-attachment regions.J Cell Sci. 2004 Mar 1;117(Pt 7):999-1008. doi: 10.1242/jcs.00976. J Cell Sci. 2004. PMID: 14996931
-
SATB1 regulates beta-like globin genes through matrix related nuclear relocation of the cluster.Biochem Biophys Res Commun. 2009 May 22;383(1):11-5. doi: 10.1016/j.bbrc.2009.03.122. Epub 2009 Mar 28. Biochem Biophys Res Commun. 2009. PMID: 19332023
-
MARs and MARBPs: key modulators of gene regulation and disease manifestation.Subcell Biochem. 2007;41:213-30. Subcell Biochem. 2007. PMID: 17484130 Review.
-
Transcriptional augmentation: modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements).Crit Rev Eukaryot Gene Expr. 2000;10(1):73-90. Crit Rev Eukaryot Gene Expr. 2000. PMID: 10813396 Review.
Cited by
-
The Satb1 protein directs hematopoietic stem cell differentiation toward lymphoid lineages.Immunity. 2013 Jun 27;38(6):1105-15. doi: 10.1016/j.immuni.2013.05.014. Epub 2013 Jun 20. Immunity. 2013. PMID: 23791645 Free PMC article.
-
A mini review of MAR-binding proteins.Mol Biol Rep. 2010 Oct;37(7):3553-60. doi: 10.1007/s11033-010-0003-8. Epub 2010 Feb 22. Mol Biol Rep. 2010. PMID: 20174991 Review.
-
Isolation and characterization of SATB2, a novel AT-rich DNA binding protein expressed in development- and cell-specific manner in the rat brain.Neurochem Res. 2006 Feb;31(2):237-46. doi: 10.1007/s11064-005-9012-8. Epub 2006 Apr 4. Neurochem Res. 2006. PMID: 16604441
-
Involvement of the SATB1/F-actin complex in chromatin reorganization during active cell death.Int J Mol Med. 2014 Jun;33(6):1441-50. doi: 10.3892/ijmm.2014.1710. Epub 2014 Mar 21. Int J Mol Med. 2014. PMID: 24676287 Free PMC article.
-
MicroRNA regulation in cancer-associated fibroblasts.Cancer Immunol Immunother. 2012 Feb;61(2):231-237. doi: 10.1007/s00262-011-1139-7. Epub 2011 Nov 16. Cancer Immunol Immunother. 2012. PMID: 22083346 Free PMC article. Review.
References
-
- Berezney R. and Coffey, D.S. 1974. Identification of a nuclear protein matrix. Biochem. Biophys. Res. Commun. 60: 1410-1417. - PubMed
-
- Best J.L., Ganiatsas, S., Agarwal, S., Changou, A., Salomoni, P., Shirihai, O., Meluh, P.B., Pandolfi, P.P., and Zon, L.I. 2002. SUMO-1 protease-1 regulates gene transcription through PML. Mol. Cell 10: 843-855. - PubMed
-
- Bode J., Benham, C., Knopp, A., and Mielke, C. 2000. Transcriptional augmentation: Modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements). Crit. Rev. Eukaryot. Gene Expr. 10: 73-90. - PubMed
-
- Cai S., Han, H.J., and Kohwi-Shigematsu, T. 2003. Tissue-specific nuclear architecture and gene expression regulated by SATB1. Nat. Genet. 34: 42-51. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
