Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia
- PMID: 13679810
- PMCID: PMC7126607
- DOI: 10.1016/s0091-6749(03)01799-8
Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia
Abstract
Background: The main symptoms of primary ciliary dyskinesia (PCD) are nasal rhinorrhea or blockage and moist-sounding cough. Diagnosis can be difficult and is based on an abnormal ciliary beat frequency, accompanied by specific abnormalities of the ciliary axoneme. It is unknown whether determining ciliary beat pattern related to specific ultrastructural ciliary defects might help in the diagnosis of PCD.
Objective: We sought to determine ciliary beat pattern and beat frequency (CBF) associated with the 5 common ultrastructural defects responsible for PCD.
Methods: Nasal brushings were performed on 56 children with PCD. Ciliary movement was recorded using digital high-speed video imaging to assess beat frequency and pattern. Electron microscopy was performed.
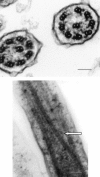
Results: In patients with an isolated outer dynein arm or with an outer and inner dynein arm defect, 55% and 80% of cilia were immotile, respectively. Cilia that moved were only flickering. Mean CBF (+/- 95% CI) was 2.3 Hz (+/- 1.2) and 0.8 Hz(+/- 0.8), respectively. Cilia with an isolated inner dynein arm or a radial spoke defect had similar beat patterns. Cilia appeared stiff, had a reduced amplitude, and failed to bend along their length. Immotile cilia were present in 10% of cilia with an inner dynein arm defect and in 30% of radial spoke defects. Mean CBF was 9.3 Hz (+/- 2.6) and 6.0 Hz (+/- 3.1), respectively. The ciliary transposition defect produced a large circular beat pattern (mean CBF, 10.7 Hz [+/- 1.1]). No cilia were immotile.
Conclusions: Different ultrastructural defects responsible for PCD result in predictable beat patterns. Recognition of these might help in the diagnostic evaluation of patients suspected of having PCD.
Figures




Similar articles
-
Primary ciliary dyskinesia: evaluation using cilia beat frequency assessment via spectral analysis of digital microscopy images.J Appl Physiol (1985). 2011 Jul;111(1):295-302. doi: 10.1152/japplphysiol.00629.2010. Epub 2011 May 5. J Appl Physiol (1985). 2011. PMID: 21551013 Free PMC article.
-
Cilia motility and structure in primary and secondary ciliary dyskinesia.Am J Rhinol Allergy. 2010 May-Jun;24(3):175-80. doi: 10.2500/ajra.2010.24.3448. Am J Rhinol Allergy. 2010. PMID: 20537282
-
The challenges of diagnosing primary ciliary dyskinesia.Proc Am Thorac Soc. 2011 Sep;8(5):434-7. doi: 10.1513/pats.201103-028SD. Proc Am Thorac Soc. 2011. PMID: 21926395 Free PMC article. Review.
-
Inner dynein arm defects causing primary ciliary dyskinesia: repeat testing required.Eur Respir J. 2011 Sep;38(3):603-7. doi: 10.1183/09031936.00108410. Epub 2011 Mar 15. Eur Respir J. 2011. PMID: 21406509
-
[Nasal ciliary investigations for the diagnosis of primary ciliary dyskinesia in children].Arch Pediatr. 2004 Apr;11(4):390-3. doi: 10.1016/j.arcped.2003.11.030. Arch Pediatr. 2004. PMID: 15051102 Review. French.
Cited by
-
Diagnosis of primary ciliary dyskinesia.J Bras Pneumol. 2015 May-Jun;41(3):251-63. doi: 10.1590/S1806-37132015000004447. J Bras Pneumol. 2015. PMID: 26176524 Free PMC article. Review.
-
Mutations of DNAI1 in primary ciliary dyskinesia: evidence of founder effect in a common mutation.Am J Respir Crit Care Med. 2006 Oct 15;174(8):858-66. doi: 10.1164/rccm.200603-370OC. Epub 2006 Jul 20. Am J Respir Crit Care Med. 2006. PMID: 16858015 Free PMC article.
-
Intracellular Cl- Regulation of Ciliary Beating in Ciliated Human Nasal Epithelial Cells: Frequency and Distance of Ciliary Beating Observed by High-Speed Video Microscopy.Int J Mol Sci. 2020 Jun 5;21(11):4052. doi: 10.3390/ijms21114052. Int J Mol Sci. 2020. PMID: 32517062 Free PMC article. Review.
-
Airway Epithelial Cell Cilia and Obstructive Lung Disease.Cells. 2016 Nov 11;5(4):40. doi: 10.3390/cells5040040. Cells. 2016. PMID: 27845721 Free PMC article. Review.
-
Primary Ciliary Dyskinesia Patient-Specific hiPSC-Derived Airway Epithelium in Air-Liquid Interface Culture Recapitulates Disease Specific Phenotypes In Vitro.Cells. 2023 May 24;12(11):1467. doi: 10.3390/cells12111467. Cells. 2023. PMID: 37296588 Free PMC article.
References
-
- Turner JA, Corkey CW, Lee JY, Levison H, Surgess J. Clinical expression of immotile cilia syndrome. Pediatrics. 1981;67:805–810. - PubMed
-
- Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. Eur Resp J. 1997;10:2376–2379. - PubMed
-
- Bush A, Cole PJ, Hariri M, Mackay I, Phillips G, O'Callaghan C. Primary ciliary dyskinesia: diagnosis and standards of care. Eur Respir J. 1998;12:982–988. - PubMed
-
- Schidlow DV. Primary ciliary dyskinesia (the immotile cilia syndrome) Ann Allergy. 1994;73:457–468. - PubMed
-
- Meeks M, Bush A. Primary ciliary dyskinesia. Pediatr Pulmonol. 2000;29:307–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

