Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation
- PMID: 12867438
- PMCID: PMC165770
- DOI: 10.1128/JB.185.15.4305-4314.2003
Chill induction of the SigB-dependent general stress response in Bacillus subtilis and its contribution to low-temperature adaptation
Abstract
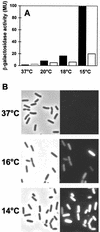
A variety of environmental and metabolic cues trigger the transient activation of the alternative transcription factor SigB of Bacillus subtilis, which subsequently leads to the induction of more than 150 general stress genes. This general stress regulon provides nongrowing and nonsporulated cells with a multiple, nonspecific, and preemptive stress resistance. By a proteome approach we have detected the expression of the SigB regulon during continuous growth at low temperature (15 degrees C). Using a combination of Western blot analysis and SigB-dependent reporter gene fusions, we provide evidence for high-level and persistent induction of the sigB operon and the SigB regulon, respectively, in cells continuously exposed to low temperatures. In contrast to all SigB-activating stimuli described thus far, induction by low temperatures does not depend on the positive regulatory protein RsbV or its regulatory phosphatases RsbU and RsbP, indicating the presence of an entirely new pathway for the activation of SigB by chill stress in B. subtilis. The physiological importance of the induction of the general stress response for the adaptation of B. subtilis to low temperatures is emphasized by the observation that growth of a sigB mutant is drastically impaired at 15 degrees C. Inclusion of the compatible solute glycine betaine in the growth medium not only improved the growth of the wild-type strain but rescued the growth defect of the sigB mutant, indicating that the induction of the general stress regulon and the accumulation of glycine betaine are independent means by which B. subtilis cells cope with chill stress.
Figures




Similar articles
-
RsbV-independent induction of the SigB-dependent general stress regulon of Bacillus subtilis during growth at high temperature.J Bacteriol. 2004 Sep;186(18):6150-8. doi: 10.1128/JB.186.18.6150-6158.2004. J Bacteriol. 2004. PMID: 15342585 Free PMC article.
-
Comprehensive characterization of the contribution of individual SigB-dependent general stress genes to stress resistance of Bacillus subtilis.J Bacteriol. 2005 Apr;187(8):2810-26. doi: 10.1128/JB.187.8.2810-2826.2005. J Bacteriol. 2005. PMID: 15805528 Free PMC article.
-
The response of Bacillus licheniformis to heat and ethanol stress and the role of the SigB regulon.Proteomics. 2013 Jul;13(14):2140-61. doi: 10.1002/pmic.201200297. Proteomics. 2013. PMID: 23592518
-
Non-specific, general and multiple stress resistance of growth-restricted Bacillus subtilis cells by the expression of the sigmaB regulon.Mol Microbiol. 1998 Sep;29(5):1129-36. doi: 10.1046/j.1365-2958.1998.00977.x. Mol Microbiol. 1998. PMID: 9767581 Review.
-
SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria.Annu Rev Microbiol. 2007;61:215-36. doi: 10.1146/annurev.micro.61.080706.093445. Annu Rev Microbiol. 2007. PMID: 18035607 Review.
Cited by
-
Exposure of Bacillus subtilis to low pressure (5 kilopascals) induces several global regulons, including those involved in the SigB-mediated general stress response.Appl Environ Microbiol. 2014 Aug;80(16):4788-94. doi: 10.1128/AEM.00885-14. Epub 2014 May 30. Appl Environ Microbiol. 2014. PMID: 24878601 Free PMC article.
-
6S-2 RNA deletion in the undomesticated B. subtilis strain NCIB 3610 causes a biofilm derepression phenotype.RNA Biol. 2021 Jan;18(1):79-92. doi: 10.1080/15476286.2020.1795408. Epub 2020 Aug 30. RNA Biol. 2021. PMID: 32862759 Free PMC article.
-
Genetic control of osmoadaptive glycine betaine synthesis in Bacillus subtilis through the choline-sensing and glycine betaine-responsive GbsR repressor.J Bacteriol. 2012 May;194(10):2703-14. doi: 10.1128/JB.06642-11. Epub 2012 Mar 9. J Bacteriol. 2012. PMID: 22408163 Free PMC article.
-
SigmaB-dependent and sigmaB-independent mechanisms contribute to transcription of Listeria monocytogenes cold stress genes during cold shock and cold growth.Appl Environ Microbiol. 2007 Oct;73(19):6019-29. doi: 10.1128/AEM.00714-07. Epub 2007 Aug 3. Appl Environ Microbiol. 2007. PMID: 17675428 Free PMC article.
-
Giving a signal: how protein phosphorylation helps Bacillus navigate through different life stages.FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad044. doi: 10.1093/femsre/fuad044. FEMS Microbiol Rev. 2023. PMID: 37533212 Free PMC article. Review.
References
-
- Alper, S., A. Dufour, D. A. Garsin, L. Duncan, and R. Losick. 1996. Role of adenosine nucleotides in the regulation of a stress-response transcription factor in Bacillus subtilis. J. Mol. Biol. 260:165-177. - PubMed
-
- Antelmann, H., J. Bernhardt, R. Schmid, H. Mach, U. Völker, and M. Hecker. 1997. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis 18:1451-1463. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

