Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS
- PMID: 12805295
- PMCID: PMC6740816
- DOI: 10.1523/JNEUROSCI.23-11-04549.2003
Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS
Abstract
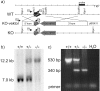
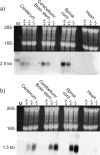
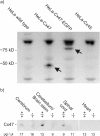
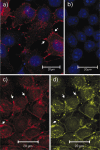
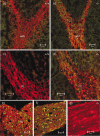
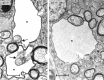
To further characterize the recently described gap junction gene connexin 47 (Cx47), we generated Cx47-null mice by replacing the Cx47 coding DNA with an enhanced green fluorescent protein (EGFP) reporter gene, which was thus placed under control of the endogenous Cx47 promoter. Homozygous mutant mice were fertile and showed no obvious morphological or behavioral abnormalities. Colocalization of EGFP fluorescence and immunofluorescence of cell marker proteins revealed that Cx47 was mainly expressed in oligodendrocytes in highly myelinated CNS tissues and in few calcium-binding protein S100beta subunit-positive cells but not in neurons or peripheral sciatic nerve. This corrects our previous conclusion that Cx47 mRNA is expressed in brain and spinal cord neurons (Teubner et al., 2001). Cx47 protein was detected by Western blot analysis after immunoprecipitation in CNS tissues of wild-type mice but not in heart or Cx47-deficient tissues. Electron microscopic analysis of CNS white matter in Cx47-deficient mice revealed a conspicuous vacuolation of nerve fibers, particularly at the site of the optic nerve where axons are first contacted by oligodendrocytes and myelination starts. Initial analyses of Cx32/Cx47-double-deficient mice showed that these mice developed an action tremor and died on average at 51 d after birth. The central white matter of these double-deficient mice exhibited much more abundant vacuolation in nerve fibers than mice deficient only in Cx47.
Figures





Similar articles
-
Panglial gap junctional communication is essential for maintenance of myelin in the CNS.J Neurosci. 2012 May 30;32(22):7499-518. doi: 10.1523/JNEUROSCI.0392-12.2012. J Neurosci. 2012. PMID: 22649229 Free PMC article.
-
Connexins are critical for normal myelination in the CNS.J Neurosci. 2003 Jul 2;23(13):5963-73. doi: 10.1523/JNEUROSCI.23-13-05963.2003. J Neurosci. 2003. PMID: 12843301 Free PMC article.
-
Gene therapy targeting oligodendrocytes provides therapeutic benefit in a leukodystrophy model.Brain. 2017 Mar 1;140(3):599-616. doi: 10.1093/brain/aww351. Brain. 2017. PMID: 28100454 Free PMC article.
-
Connexin 43/47 channels are important for astrocyte/ oligodendrocyte cross-talk in myelination and demyelination.J Biosci. 2018 Dec;43(5):1055-1068. doi: 10.1007/s12038-018-9811-0. J Biosci. 2018. PMID: 30541963 Free PMC article. Review.
-
Severe Convulsions and Dysmyelination in Both Jimpy and Cx32/47 -/- Mice may Associate Astrocytic L-Channel Function with Myelination and Oligodendrocytic Connexins with Internodal Kv Channels.Neurochem Res. 2017 Jun;42(6):1747-1766. doi: 10.1007/s11064-017-2194-z. Epub 2017 Feb 18. Neurochem Res. 2017. PMID: 28214987 Review.
Cited by
-
Activated immune response in an inherited leukodystrophy disease caused by the loss of oligodendrocyte gap junctions.Neurobiol Dis. 2015 Oct;82:86-98. doi: 10.1016/j.nbd.2015.05.018. Epub 2015 Jun 4. Neurobiol Dis. 2015. PMID: 26051537 Free PMC article.
-
Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus.Neuroscience. 2007 Jul 29;147(4):938-56. doi: 10.1016/j.neuroscience.2007.04.061. Epub 2007 Jul 2. Neuroscience. 2007. PMID: 17601673 Free PMC article.
-
Astrocyte and oligodendrocyte connexins of the glial syncytium in relation to astrocyte anatomical domains and spatial buffering.Cell Commun Adhes. 2003 Jul-Dec;10(4-6):401-6. doi: 10.1080/15419060390263191. Cell Commun Adhes. 2003. PMID: 14681048 Free PMC article.
-
Emerging cellular themes in leukodystrophies.Front Cell Dev Biol. 2022 Aug 8;10:902261. doi: 10.3389/fcell.2022.902261. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36003149 Free PMC article. Review.
-
Pathologic and phenotypic alterations in a mouse expressing a connexin47 missense mutation that causes Pelizaeus-Merzbacher-like disease in humans.PLoS Genet. 2011 Jul;7(7):e1002146. doi: 10.1371/journal.pgen.1002146. Epub 2011 Jul 7. PLoS Genet. 2011. PMID: 21750683 Free PMC article.
References
-
- Barger SW, Wolchok SR, Van Eldik LJ ( 1992) Disulfide-linked S100 beta dimers and signal transduction. Biochim Biophys Acta 1160: 105–112. - PubMed
-
- Bosio A, Büssow H, Adam J, Stoffel W ( 1998) Galactosphingolipids and axono-glial interaction in myelin of the central nervous system. Cell Tissue Res 292: 199–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
